Astrogliosis and neuroinflammation underlie scoliosis upon cilia dysfunction
Figures
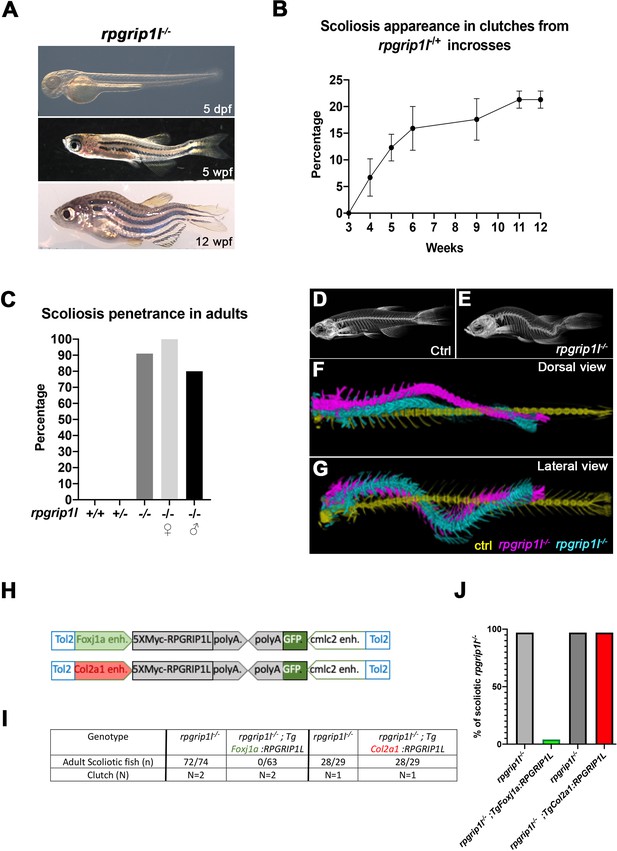
rpgrip1l-/- juvenile fish develop scoliosis, which is rescued by RPGRIP1L expression in foxj1a-positive cells.
(A) Representative rpgrip1l-/- fish at 5 days post-fertilization (dpf) (4 mm body length, larvae), 5 weeks pf (wpf) (1.2 cm body length, juveniles) and 12 wpf (2.5 cm body length, adults), showing absence of morphological defects in embryos, onset of spine curvature (tail-up) in juveniles and scoliosis in adults. (B, C) Graph showing the time course of scoliosis appearance (B) in rpgrip1l-/+ incrosses (total 4 clutches, 252 fish) and scoliosis penetrance (C) in adults. (D, E) Micro-computed tomography (μCT) scans of control siblings (D) and rpgrip1l-/- (E) adult fish. Four fish were analyzed for each condition. (F, G) Dorsal and lateral superimposed μCT views of the spines of one control (yellow) and two rpgrip1l-/- (pink, cyan) adult fish illustrating the 3D spine curvatures in mutants. (H) Schematic representation of the two rescue constructs. (I) Table presenting the number and phenotype of rpgrip1l-/- fish generated for both rescue experiments by stable transgenesis (J) Graph representing scoliosis penetrance in transgenic and non-transgenic rpgrip1l-/- siblings.
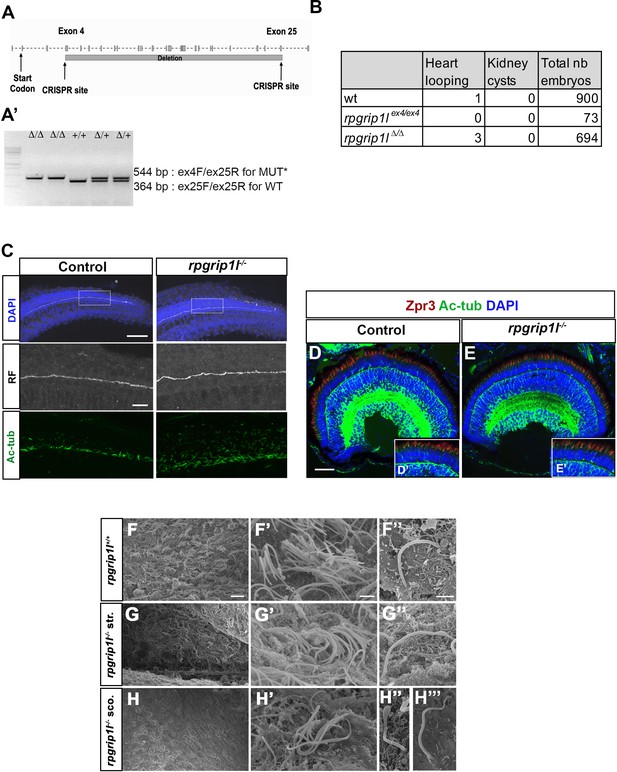
Production and characterization of the rpgrip1∆ and rpgrip1lex4 fish lines.
(A) Schematic exon-intron structure of the Danio rerio rpgrip1l gene. Two alleles were produced by CRISPR/cas9-mediated genome engineering, rpgrip1lex4 with a nonsense mutation in exon 4 and rpgrip1l∆4-25 (also called rpgrip1l∆ or rpgrip1l-) with a large deletion between exons 4 and 25, encompassing most of the protein coding sequence. Both alleles had identical embryonic phenotype (panel B and data not shown). (A’) Electrophoresis gel showing the migration profile of amplicons for each genotype, rpgrip1lΔ/Δ, rpgrip1lΔ/+, rpgrip1+l/+ produced by triplex PCR (B) Absence of laterality defects and kidney cysts in rpgrip1lΔ/Δ and rpgrip1lex4/ex4 embryos. (C) Immunostaining on whole 30 hpf control (left panels) and rpgrip1l-/- (right panels) embryos to visualize the RF (anti-RF antibody, middle panels) and cilia (acetylated tubulin antibody, lower panels) in the caudal region. Nuclei (upper panels) are stained with DAPI. The RF is present in the central canal of rpgrip1l-/- embryos, as well as cilia in the whole neural tube width. Scale bar: 6 µm for upper panels; 15 µm for lower panels. (D, E) Absence of retinal morphological defects in 5 dpf rpgrip1l-/- larvae. Retinal sections of control (D) and rpgrip1l-/- (E) larvae were immunostained with Zpr3 (red) to label the external segment of photoreceptors and Acetylated Tubulin (Ac Tub) (green) to label axonemes. (D’) and (E’) are insets in (D) and (E) focusing on the photoreceptor layer. Scale bar: 50 μm. (F-H’’’) Scanning electron microscopy of brain ventricles in control (F-F’’), straight (str.) rpgrip1l-/- (G-G’’) and scoliotic (sco.) rpgrip1l-/- (H-H’’’) adult (3 months) fish. Ependymal multiciliated cells of the rhombencephalic ventricle (RhV) are almost totally absent in rpgrip1l-/- scoliotic fish (H-H'), while they are present in straight rpgrip1l-/-(G-G') and controls (F-F'). Cilia of hindbrain mono-ciliated cells in rpgrip1l-/- (F’’, G’’) and controls (G’’). Scale bars: 10 μm for (F-I), 2 μm for F’-H’, 1 μm for F’’, G’’, H‘’ and H’’’.
-
Figure 1—figure supplement 1—source data 1
Raw and unedited rpgrip1l genotyping gel showing wild-type and mutants amplicons from a triplex PCR.
- https://cdn.elifesciences.org/articles/96831/elife-96831-fig1-figsupp1-data1-v1.tiff
-
Figure 1—figure supplement 1—source data 2
Edited rpgrip1l genotyping gel showing the cropped region.
- https://cdn.elifesciences.org/articles/96831/elife-96831-fig1-figsupp1-data2-v1.tiff
Micro-CT of juvenile spines.
Superposition of 3D-reconstructed spines of 5 wpf juvenile fish, 1 control (white), 3 rpgrip1l∆/∆ of different severities: 2 tail-up (yellow, blue), and 1 scoliotic (pink).
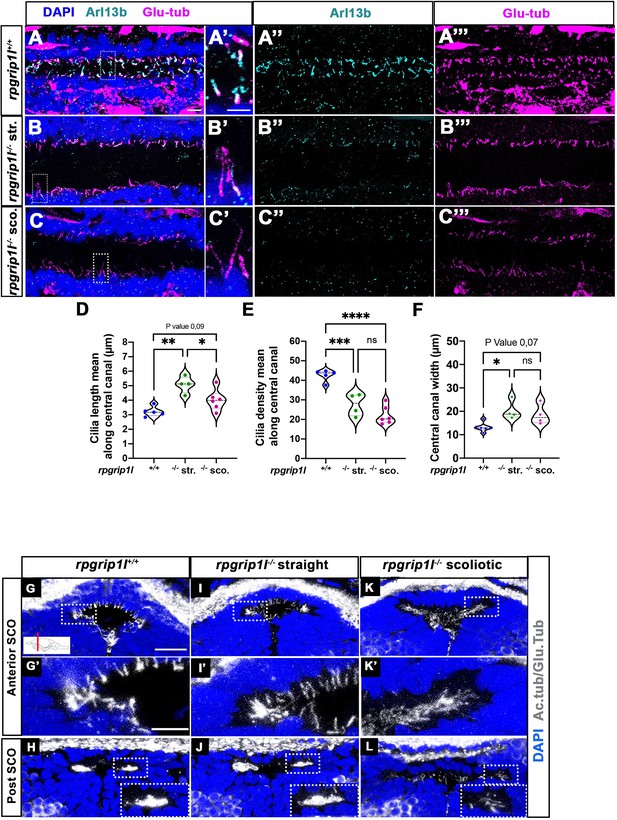
Altered ciliogenesis at trunk and subcommissural organ (SCO) levels.
(A-C’’’) Immunostaining of trunk sagittal sections at the level of the spinal cord central canal in rpgrip1l+/+ (A-A’’’; n=5), straight rpgrip1l-/- (B-B’’’; n=4) and scoliotic rpgrip1l-/- fish (C-C’’’; n=6) at 8 weeks (N=2 independent experiments). Cilia were immunostained with Arl13b (cyan, A’’, B’’, C’’) and glutamylated-tubulin (magenta, A’’’, B’’’, C’’’). Panels A-C’ are merged images with DAPI. Panels A’, B’ and C’ show higher magnification of the squared regions in A, B and C, respectively. Scale bars: 10 μm in A, 5 μm in A’. (D) Violin plot showing the distribution of cilia length along the central canal of rpgrip1l+/+ siblings (n=300 cilia from 5 fish), straight rpgrip1l-/- (n=267 from 4 fish) and scoliotic rpgrip1l-/- (n=414 from 6 fish) juvenile fish (N=2). (E) Violin plot showing the distribution of cilia density along the central canal of juvenile rpgrip1l+/+ siblings (n=5), straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- fish (n=6) (N=2). Density is the average number of ventral and dorsal cilia lining the CC over a length of 100 µm from three to five sections for each fish. Each dot represents the mean cilia length (D) or cilia density (E) of four to eight sections analyzed at different antero-posterior levels. Statistical analysis was performed using Tukey’s multiple comparisons test where * means p-value <0.05; ** means p-value <0.01; *** means p-value <0.001; **** means p-value <0.0001. (F) Violin plot showing the distribution of width of the central canal (nuclear-free region) (in µm) of juvenile rpgrip1l+/+ (n=5), straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- fish (n=6) (N=2). Each dot represents the mean of 3 measurements made at different AP levels. Statistical analysis was performed using Tukey’s multiple comparisons test where ns means non-significant, * means p-value <0.05. (G–L) Immunostaining of cilia on transverse sections of the brain at SCO level using glutamylated-tubulin and acetylated-tubulin antibodies (both in white), in rpgrip1l+/+ (n=6) (G, G’, H), straight rpgrip1l-/- (n=3) (I, I’, J) and scoliotic rpgrip1l-/- (n=7) (K, K’, L) 8 wpf fish (N=2). Nuclei were stained with DAPI (blue). G’, I’ and K’ are higher magnifications of squared regions in G, I and K, respectively. In H, J and L the bottom-right inset represents a higher magnification of the squared region. Scale bars in G and G’: 10 µm.
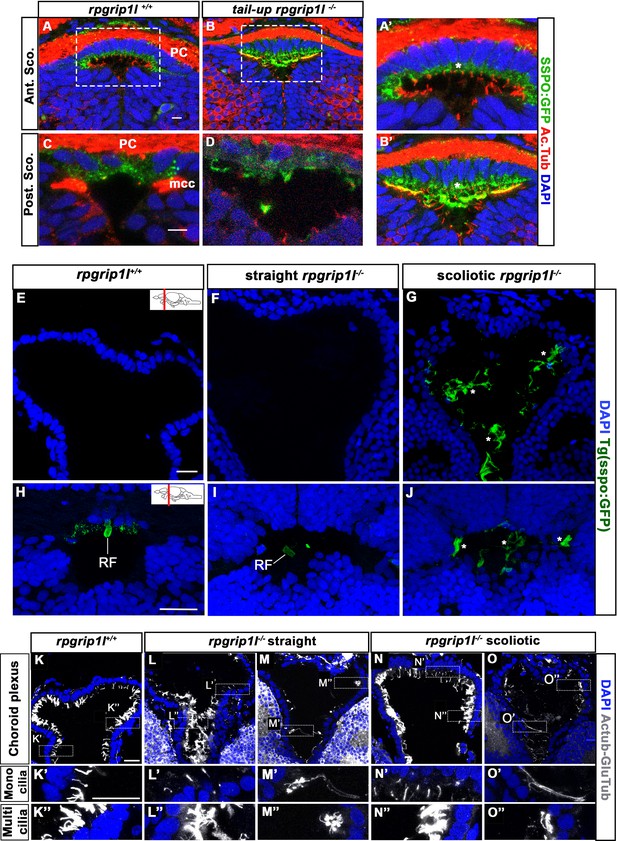
Presence of scospondin aggregates and heterogenous ciliary defects in the fChP of rpgrip1l-/- juvenile fish.
(A-B’) Immunostaining on transverse sections of the SCO, at anterior level (A, A’, B) or posterior level (C, D) in rpgrip1l+/+ (n=3) and tail-up rpgrip1l-/- (n=3), transgenic for Sspo-GFP (N=2). Sspo-GFP forms small cytoplasmic aggregates within SCO cells of both genotypes (* in A’ and B’), under the primary cilia extending towards the DiV, and accumulates at the apical surface of tail-up rpgrip1l-/-, creating a double positive area for cilia and Sspo-GFP staining (yellow colour in B’). Multiciliated cells lateral to the posterior SCO (mcc) are negative for Sspo-GFP staining in controls (C) and absent in tail-up rpgrip1l-/- (D). (E–J) GFP immunostaining on transverse section of the fChP (E–G) or at the level of the optic tectum (H–J) in rpgrip1l+/+ (n=7), straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- (n=5) 8 wpf fish transgenic for sspo-GFP (N=2). Scoliotic rpgrip1l-/- fish present with filamentous material and aggregates within the ventricles (G, J) while a small RF fragment is present within the ventricle of controls (H) and straight mutants (I) at optic tectum level. * indicates aggregates. (K-O’’) Representative maximum intensity Z-stack projections of confocal transverse brain section at the level of the fChP in rpgrip1l+/+ (K-K’’) (n=7), straight rpgrip1l-/- (L-M’’) (n=4) and scoliotic rpgrip1l-/- (N-O’’) (n=5) 8 wpf fish (N=2). Cilia were stained with glutamylated-tubulin and acetylated-tubulin antibodies (both in white) and nuclei were stained with DAPI (blue). Sections shown are at the level of the habenular nuclei. At this level, monocilia in the dorsal midline region (K’) and multicilia on lateral sides (K’’) (n=7/7) are present in controls. Straight rpgrip1l-/- exhibited either normal (L’, L’’) (n=2/4) or abnormal and sparser (M’, M’’) (n=2/4) monocilia and multicilia. Similarly, scoliotic rpgrip1l-/- presented either normal monocilia and multicilia (N’, N’’) (n=3/5) or long monocilia (O’) and decreased cilia density of multi-ciliated tufts (O’’) (n=2/5). Scale bars: 20 µm.
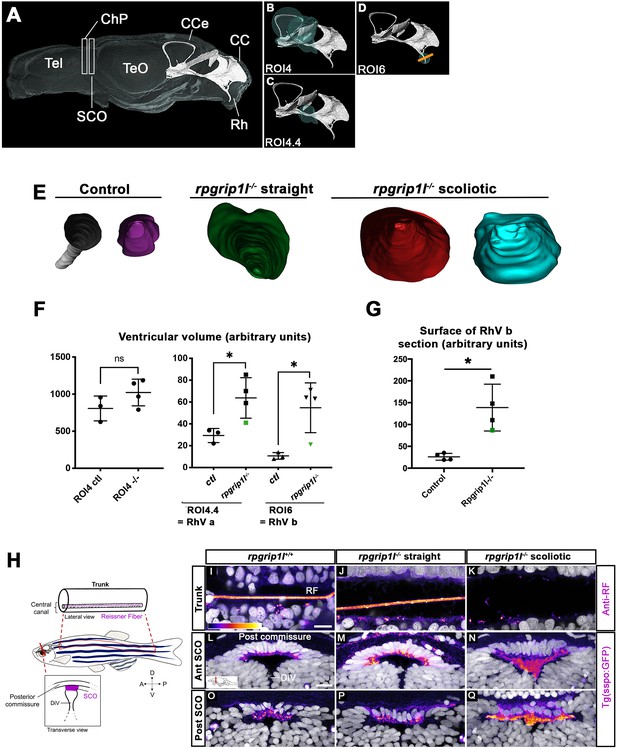
rpgrip1 -/- juveniles show ventricular dilations and loss of the Reissner Fiber at scoliosis onset.
(A) Reconstruction of the RhV at the level of posterior midbrain and hindbrain in a transparised 5 wpf control zebrafish brain, stained with ZO1 antibody (ventricular surface) and DiI (global brain shape). Several brain regions are annotated on the reconstruction where Tel means telencephalon, ChP forebrain choroid plexus, SCO subcommissural organ, TeO optic tectum, CCe corpus cerebelli, CC crista cerebellaris and Rh rhombencephalon. (B–D) Cyan circles represent the ROIs measured in F. The orange line in D indicates the level of optical sections in E. (E) Optical transverse sections showing the caudal part of reconstructed ventricles of a control and two rpgrip1l-/- fish, one straight and two tail-up. (F) Graphs of the ventricle volume at the onset of scoliosis in three control and four rpgrip1l-/- (one straight and three tail-up) fish. The green dot corresponds to the straight mutant fish. Each dot represents a fish. Statistical analysis was performed using unpaired t-test where ns means non-significant, and * means p-value <0.05. (G) Graph of the surface of the optical sections as illustrated in E. The green dot corresponds to a straight mutant fish. Each dot represents a fish. Statistical analysis was performed using unpaired t-test where ns means non-significant, and * means p-value <0.05. (H) Schematic representation of a portion of the spinal cord central canal in a lateral view of the fish trunk, showing the Reissner Fiber (RF), and of the SCO in a transverse view of the brain at the level of diencephalic Ventricle (DiV). (I–Q) Visualization of the GFP fluorescence in the sspo-GFPuts24/+ transgenic line (fire LUT) in sagittal sections of the trunk (I–K) (N=3) and transverse sections of the brain at the level of the SCO (N=2) (L–Q) in juvenile fish. The corresponding fish are 8 wpf rpgrip1l+/+ (I, L, O) (n=10); straight rpgrip1l-/- (J, M, P) (n=9) and scoliotic rpgrip1l-/- (K, N, Q) (n=8). L-N and O-Q show sections at anterior and posterior SCO levels, respectively. Scale bars: 5 µm in I-K, 10 μm in L-Q.
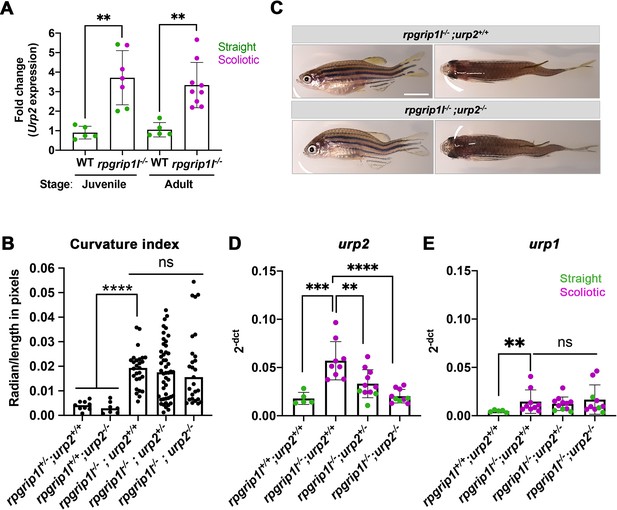
Upregulation of urp1/2 expression does not contribute to axis curvature of rpgrip1l-/- fish.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) assaying urp2 expression level in rpgrip1l-/- and rpgrip1l+/+ (WT) siblings. Each dot represents the value obtained for one fish. Green and purple dots represent straight and scoliotic rpgrip1l-/- fish, respectively. qRT-PCR analysis was performed at 5 wpf (juvenile stage, 5 controls and 7 rpgrip1l-/-) and 12 wpf (adult stage, 5 controls and 9 rpgrip1l-/-). urp2 expression levels are increased by approximately 3.5-fold in rpgrip1l-/- at both stages. Statistical analysis was performed using unpaired t-test where **p<0.01. Error bars represent s.d. (B) Quantification of body axis curvature from dorsal and lateral views in 4 mpf fish issued from [rpgrip1l+/-, urp2+/-] incrosses. Each point represents the value measured for one fish. Statistical analysis was performed using Tukey’s multiple comparisons test where **** p<0.0001 with error bars represent s.d. n=9 [rpgrip1l+/+; urp2+/+], 7 [rpgrip1l+/+; urp2-/-], 27 [rpgrip1l-/-; urp2+/+], 48 [rpgrip1l-/-; urp2+/-] and 27 [rpgrip1l-/-; urp2-/-] fish. (C) Representative [rpgrip1l-/-; urp2+/+] and sibling [rpgrip1l-/-; urp2-/-] fish on lateral and dorsal views at 12 wpf. Scale bar 5 mm. (D, E) Expression levels of urp2 (D) and urp1 (E) in adult fish (12 wpf). Each dot represents the value for one fish. Green and purple dots represent straight and scoliotic rpgrip1l-/- fish, respectively (n=5 [rpgrip1l+/+; urp2+/+], 9 [rpgrip1l+/+; urp2-/-], 11 [rpgrip1l-/-; urp2+/-] and 10 [rpgrip1l-/-; urp2-/-]). Each gene expression level is compared to the lsm12b housekeeping gene. Statistical analysis was performed using Tukey’s multiple comparisons test where ns means non-significant and ** p<0.01, ****; ***p<0.001; p<0.0001. Error bars represent s.d.
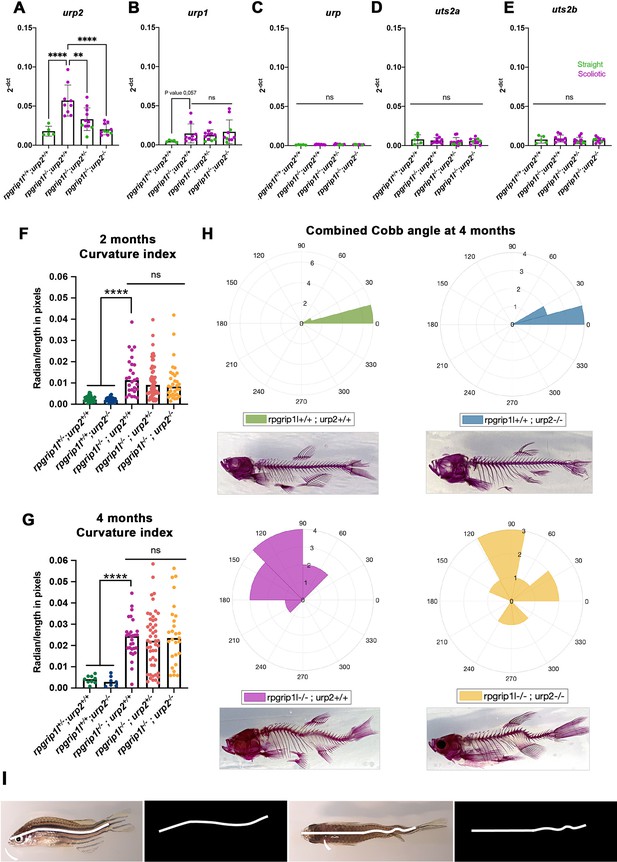
Genetic invalidation of urp2 in rpgrip1l-/- fish does not have any beneficial effect on scoliosis penetrance or severity.
(A–E) Absence of compensatory upregulation of urp family members in urp2-/- fish. Expression levels of urp2 (A), urp1 (B), urp (C), uts2a (D) and uts2b (E) in zebrafish in 13 wpf adults. Each dot represents the value measured for one fish; green and purple dots represent straight and curved fish, respectively. n=5 [rpgrip1l+/+; urp2+/+], 9 [rpgrip1l+/+; urp2-/-], 11 [rpgrip1l-/-; urp2+/-] and 10 [rpgrip1l-/-; urp2-/-]. Expression levels for each gene are compared to the lsm12b housekeeping gene. Statistical analysis was performed using the Tukey test where ns means not significant, ** p<0.01, **** p<0.0001. Error bars represent s.d. None of the uts2 family members is significantly upregulated in [rpgrip1l-/-; urp2-/-] fish compared to rpgrip1l-/-, suggesting an absence of compensation. (F, G) Quantification of body axis curvature on dorsal and lateral positions at 2 (F) and 4 (G) mpf. Each dot represents the curvature index of one fish. Statistical analysis was done using Tukey’s multiple comparisons test where ****p<0.0001, with error bars representing s.d. At 2 mpf, n=30 [rpgrip1l+/+; urp2+/+], 26 [rpgrip1l+/+; urp2-/-], 27 [rpgrip1l-/-; urp2+/+], 55 [rpgrip1l-/-; urp2+/-] and 32 [rpgrip1l-/-; urp2-/-]. At 4 mpf, n=9 [rpgrip1l+/+; urp2+/+], 7 [rpgrip1l+/+; urp2-/-], 27 [rpgrip1l-/-; urp2+/+], 48 [rpgrip1l-/-; urp2+/-] and 27 [rpgrip1l-/-; urp2-/-]. (H) Rose plots representing combined Cobb angle measurements at 4 mpf of [rpgrip1l+/+; urp2+/+] (n=7) in green, [rpgrip1l+/+; urp2-/-] (n=6) in blue, [rpgrip1l-/-; urp2+/+] (n=10) in pink and [rpgrip1l-/-; urp2-/-] (n=7) in yellow. Cobb angle measurements were performed on Alizarin red fish skeletons. One example of Alizarin red skeletons for each genotype is presented below its corresponding rose-plot. (I) Illustration of combined curvature index measurement from traced lines on lateral (left) and dorsal (right) views of fish. The curvature index of both traced lines was measured using a modified MATLAB program (LineCurvature2D function) which provided a measurement in radian/length in pixels. .
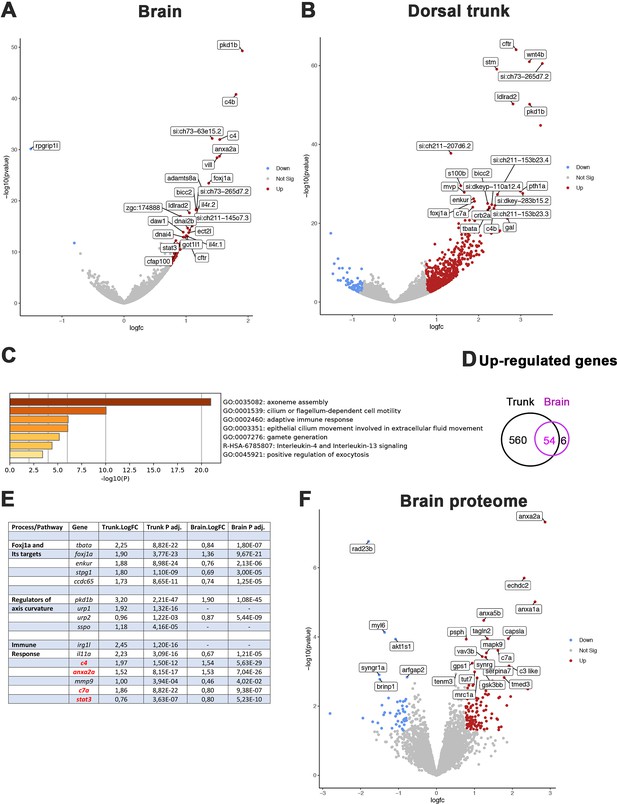
Comparative transcriptome and proteome analysis of control and rpgrip1l-/- fish.
(A, B) Volcano-plots showing differentially regulated genes in the transcriptomes of the brain (A) and dorsal trunk (B) comparing 5 controls (2 wt, 3 rpgrip1l-/+) and 7 rpgrip1l-/- (6 tail-up and 1 straight) fish at 5 wpf (0.9 cm body length). Each dot corresponds to one gene. A gene was considered deregulated if its associated P-adj. value was inferior to 0.05. Red and blue dots represent up-regulated (Log2FC >0.75), and down-regulated (Log2FC <–0.75) genes, respectively. The names of the top 25 most deregulated genes are boxed. (C) GO term analysis of biological processes in the brain samples using Metascape. (D) Venn diagram of the upregulated genes in brain and trunk samples, showing many common genes between the two regions. (E) Selected genes of interest upregulated in the trunk and/or brain of rpgrip1l-/- fish. The four genes in red were also found upregulated in the proteome of adult rpgrip1l-/- fish. (F) Volcano-plot showing differentially expressed proteins in the brain of 5 rpgrip1l+/+ fish versus 5 rpgrip1l-/- adult scoliotic fish (3 months pf). Each dot corresponds to one protein. A protein was considered differentially expressed if its associated P-adj. value was inferior to 0.05. Red and blue dots represent proteins enriched (Log2FC >0.75) or depleted (Log2FC <–0.75) in mutant brains, respectively. The names of the top 25 most differentially expressed proteins are boxed.
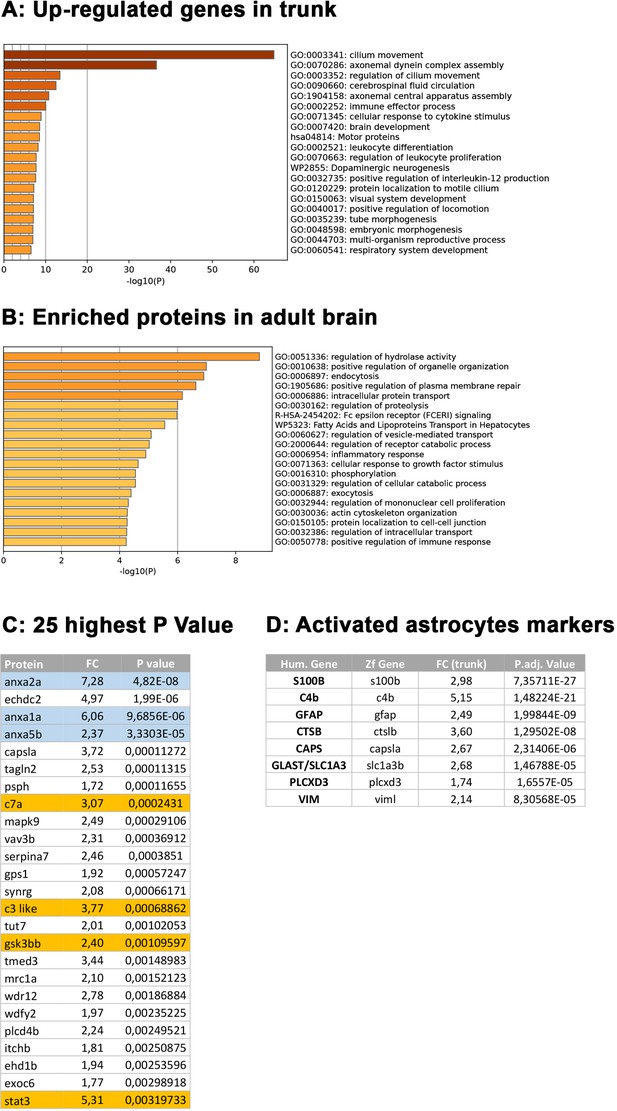
Complementary comparative transcriptome and proteomic analysis of rpgrip1l-/- versus control fish.
(A, B) GO term analysis of biological processes enriched in rpgrip1l-/- for genes upregulated in the trunk transcriptome data (A) or enriched in rpgrip1l-/- for proteins in the brain adult proteome data (B) using Metascape. (C) List of the top 25 most enriched proteins in the rpgrip1l-/- vs control brain proteome. (D) List of selected genes upregulated in the zebrafish rpgrip1l trunk transcriptome and found in different reactive astrogliosis models in single cell transcriptome studies (Matusova et al., 2023).
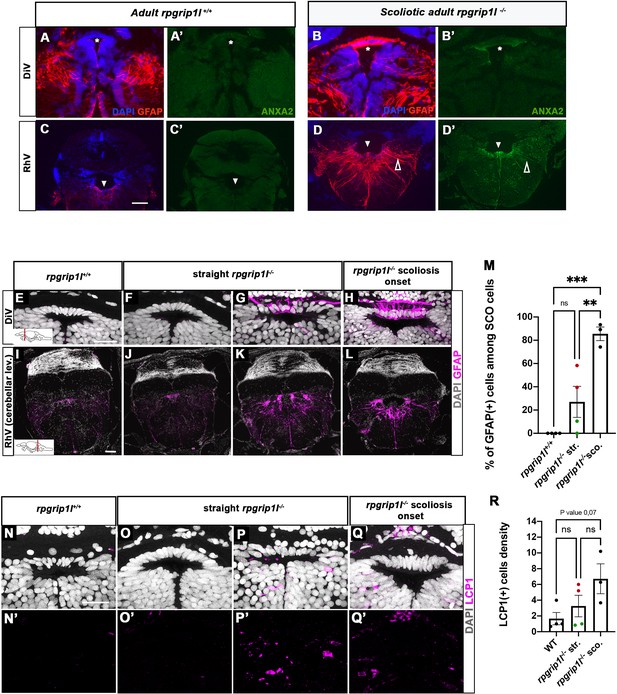
Anxa2 and GFAP upregulation and increased number of LCP1-positive cells around CNS ventricles of rpgrip1l-/- juvenile fish at scoliosis onset.
(A-D’) Immunostaining for GFAP (A, B, C, D) and Anxa2 (A’, B’, C’, D’) on adult brain transverse sections at DiV (A-B’) and RhV at cerebellar level (C-D’) levels in controls (A, A’, C, C’) (n=3) and rpgrip1l-/- scoliotic (B, B’, D, D’) (n=4) fish. Nuclei are stained with DAPI (blue). White stars indicate the SCO, white triangles point to the ventral RhV region. Open arrows point to a long cellular process co-labeled by Anxa2 and GFAP. (E–L) Immunostaining for GFAP (magenta) on transverse sections of the brain at SCO (E–H) and RhV (I–L) levels in controls (E, I) (n=4), straight rpgrip1l-/- (F, G, J, K) (n=4) and scoliotic rpgrip1l-/- (H, L) (n=3) juvenile (8 wpf) fish. Nuclei are stained with DAPI (white). Scale bar: 125 µM for A-D’, 20 µm for E-H and 100 µm for I-L. (M) Graph representing the percentage of GFAP-positive cells among the total number of SCO cells in control (n=4), straight rpgrip1l-/- (n=4), and scoliotic rpgrip1l-/- (n=3) fish at scoliosis onset (N=1). Each dot represents the mean ratio of GFAP-positive over -negative cells in 3–6 sections per fish. Green dots correspond to fish with no or very low percentage of GFAP + cells, red dots to fish with a high percentage of GFAP + cells. Statistical analysis was performed with Tukey’s multiple comparisons test where ns means non-significant, ** means p-value <0.01 and *** p-value <0.001. Error bars represent s.d. ‘str’: straight; ‘sco’: scoliotic. (N-Q’) Immunostaining for LCP1 +microglia/macrophages (magenta) on brain transverse sections at SCO levels in a rectangle of 100 µm x 60 µm located under the posterior commissure in controls (n=4) (N, N’), straight rpgrip1l-/- (n=4) (O-P’) and rpgrip1l-/- at scoliosis onset (n=3) (Q, Q’) 8 wpf fish (N=1). Nuclei are stained with DAPI (white). Scale bar: 20 µm. (R) Quantification of LCP1 + cell density around the SCO in controls (n=4), straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- (n=3) at 8 wpf. Twenty-three sections were counted for control, 21 sections for rpgrip1l-/- straight, and 18 sections for rpgrip1l-/- scoliotic fish. Each dot represents the mean value for one fish. Green dots correspond to the fish without any GFAP +SCO cells, red dots correspond to the fish with GFAP +SCO cells (SCO cells are defined as the dorsal cells located underneath the posterior commissure and above the diencephalic ventricle). Note that straight rpgrip1l-/- fish with GFAP +SCO cells present a higher number of LCP1 + cells. Statistical analysis was performed with Tukey’s multiple comparisons test where ns means non-significant. Error bars represent s.e.m. ‘str’:straight ‘sco’: scoliotic.
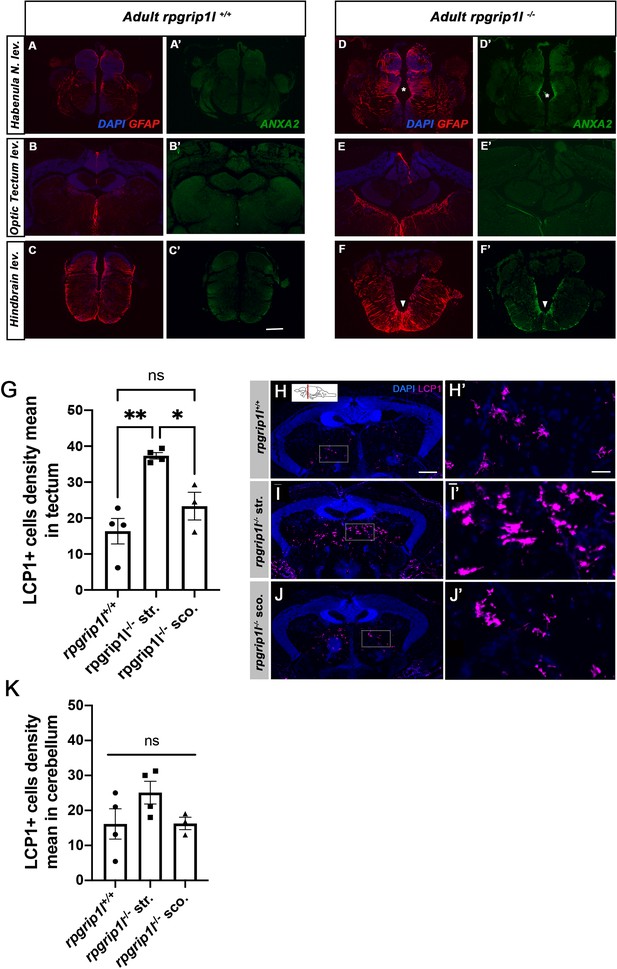
Increased GFAP and Annexin 2 staining and LCP1 + cell density in different brain regions.
(A-F’) Immunostaining of Annexin A2 (Anxa2) (A, B, C, D, E, F) and GFAP (A’, B’, C’, D’, E’, F’) on transverse brain sections at habenula (A, A’, D, D’), tectum (B, B’, E, E’) and hindbrain (C, C’, F, F’) levels in controls (A-C’) and scoliotic rpgrip1l-/- (D-F’) fish. Nuclei are stained with DAPI (blue). Scoliotic rpgrip1l-/- fish upregulate Annexin A2 and GFAP in overlapping territories. Scale bar: 20 µm. (G) Quantification of LCP1 + cell density in the optic tectum region of rpgrip1l+/+ (n=4), straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- (n=3) 8 wpf fish. Each dot represents a fish. 15–20 sections were counted for each brain level. Straight rpgrip1l-/- fish sections displayed around twice more Lcp1 + cells at tectum level than controls, while scoliotic rpgrip1l-/- fish had a slightly increased LCP1 + cell density compared to controls. Statistical analysis was performed using Tukey’s multiple comparisons test where ns means non significative and * p value <0.05, ** p value <0.01 with error bars represent s.d. (H-J’) Immunostaining of LCP1 +immune cells on transverse sections of the optic tectum region of rpgrip1l+/+ (H, H’) (n=4), straight rpgrip1l-/- (I, I’) (n=4) and scoliotic rpgrip1l-/- (J, J’) (n=3) 8 wpf fish. Immune cells are labeled with the LCP1 antibody (magenta) and nuclei with DAPI (blue). H’, I’ and J’ are magnifications of the squared regions in H, I and J, respectively. Scale bars: 100 µm for H, I, J; 30 µm for H’, I’, J’. str: straight, sco: scoliotic. (K) Quantification of LCP1 + cell density in the cerebellum region of 8 wpf fish (N=1). The analysis compared straight rpgrip1l-/- (n=4) and scoliotic rpgrip1l-/- (n=3) fish to rpgrip1l+/+ siblings (n=4). No significant difference was found between genotypes. Statistical analysis was performed using Tukey’s multiple comparisons test where ns means not significant. Error bars represent s.d. ‘str’ means straight and ‘sco’ means scoliotic.
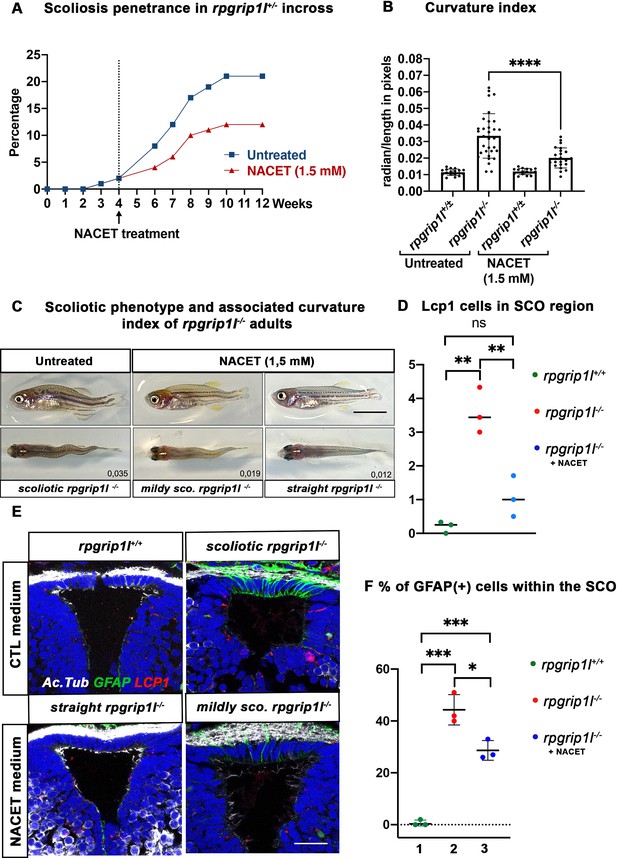
NACET treatment reduces scoliosis penetrance and severity in rpgrip1l-/- and decreases astrogliosis and LCP1 + cell number at SCO level.
(A) Kinetics of scoliosis development in rpgrip1l+/- incrosses in the presence (n=150 fish) or absence (n=174 fish) of NACET treatment (1.5 mM) from 4 to 12 weeks (N=1). ‘str’: straight; ‘sco’: scoliotic. (B) Quantification of body axis curvature from dorsal and lateral views at 13 wpf, at the end of the NACET treatment. Each dot corresponds to one fish. Among the 174 fish raised in untreated condition, we measured 18 controls (rpgrip1l±/-) and 33 rpgrip1l-/-, and upon NACET treatment, 16 controls (rpgrip1l±/+) and 23 rpgrip1l-/-. rpgrip1l±/- represents a pool of rpgrip1l+/+ and rpgrip1l+/-. Statistical analysis was performed using Tukey’s multiple comparisons test where * means p-value <0.05; ** means p-value <0.01; *** means p-value <0.001; **** means p-value <0.0001. Error bars represent s.d. (C) Representative pictures of rpgrip1l-/- fish with their curvature index without or after NACET treatment, at 13 wpf. Scale bar is 0.5 cm. Left panels correspond to an untreated rpgrip1l-/- scoliotic fish on lateral (upper) and dorsal (lower) views. Middle panels represent treated rpgrip1l-/-, with a lower curvature index than scoliotic fish on lateral (upper) and dorsal (lower) views and right panels are pictures of rescued straight rpgrip1l-/- fish on lateral (upper) and dorsal (lower) views. (D) Graph representing the number of LCP1 + cells in wt (n=3), untreated rpgrip1l-/- (n=3) and NACET-treated rgprip1l-/- (n=3) fish in the SCO region (N=1). (E) Immunostaining for GFAP (green), Acetylated tubulin (white) and LCP1 (red) on adult brain transverse sections at SCO level in untreated (upper panels) and NACET-treated (lower panels) fish. Nuclei are stained with DAPI (blue). No GFAP staining was present in controls (n=3) (upper left), while untreated mutants displayed strong GFAP labeling (upper right) (n=3). NACET treatment totally suppressed gliosis in a straight rpgrip1l-/- fish (lower right panel) (n=1) and diminished the intensity of GFAP labeling in slightly tail-up rpgrip1l-/- (n=2). Scale bar: 12.5 µm. (F) Graph presenting the percentage of GFAP positive cells among the total number of SCO secretory cells compared between controls (n=3), untreated rpgrip1l-/- (n=3), and NACET treated rpgrip1l-/- adult fish (n=3) (N=1). Each dot represents the mean of % of GFAP-positive cells present in 3–6 sections per fish.
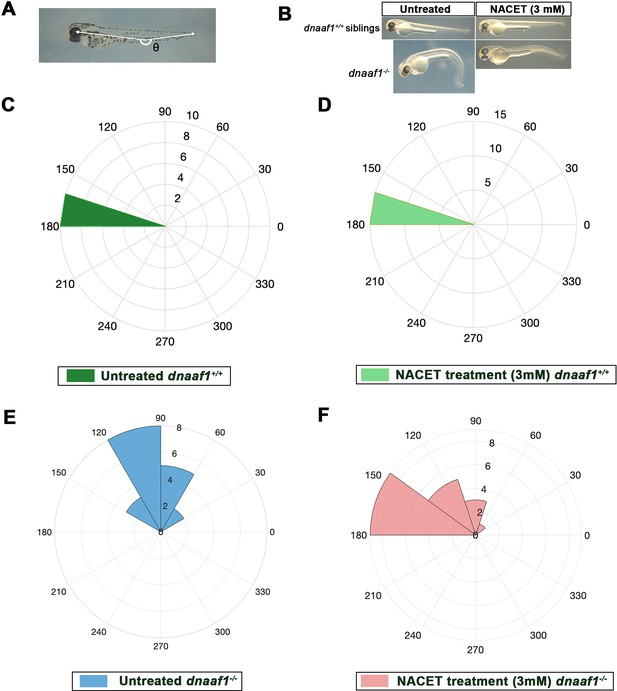
Biological assay of NACET activity on dnaaf1-/- embryos.
(A) Theta angle (θ) measurement between the eye, the caudal end of the yolk extension and the tail extremity, corresponding to the embryo curvature index. (B) Lateral views of 60 hpf representative embryos showing axial curvature phenotypes. In untreated conditions, dnaaf1-/- embryos show a characteristic curly tail-down (CTD) phenotype, whereas dnaaf1+/+ siblings have a straight axis. Treatment at 27 hpf with 3 mM NACET suppresses the CTD phenotype of dnaaf1-/- embryos and does not affect axis straightness of dnaaf1+/+ siblings. Embryos were measured around 2.5–3 mm. (C–F) Rose plots representing the (360 – θ) angle of dnaaf1+/+ and dnaaf1-/- in untreated and 3 mM NACET-treated conditions. Embryos were treated from 27hpf to 2.5 dpf and θ angles were measured at the end of the treatment. Untreated dnaaf1+/+ are represented in (C – dark green), and treated ones are in (D – light green). Untreated dnaaf1-/- are in (E – blue) and treated ones are in (F – red). NACET treatment is able to rescue curly tail down phenotype in dnaaf1-/- embryos without affecting the controls.
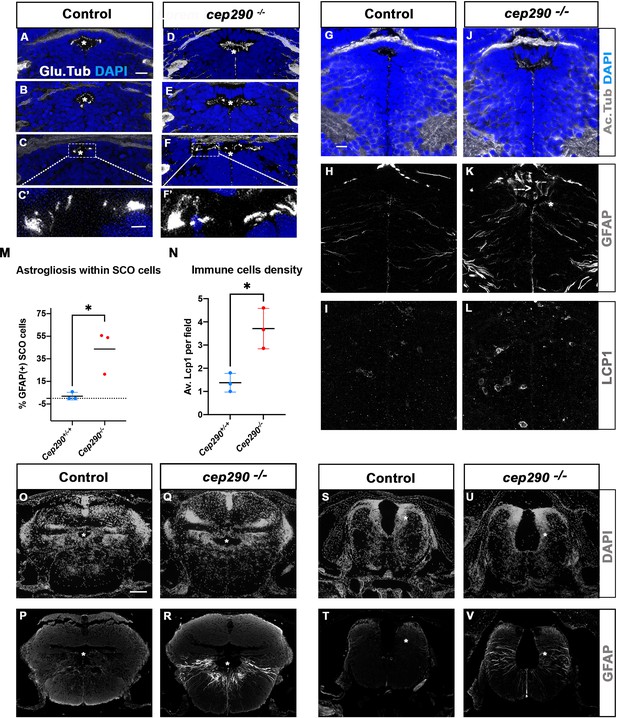
Altered ciliogenesis, GFAP upregulation and increased number of LCP1-positive cells around ventricles of cep290-/- juvenile fish at 4 wpf.
(A-F’) Immunostaining of the cilia marker glutamylated tubulin (white) on 4 wpf brain transverse sections at 3 antero-posterior SCO levels in 4 wpf control (A-C’, n=3) and cep290-/- scoliotic (D-F’, n=3) fish. Nuclei are stained with DAPI (blue). White stars indicate the diencephalic ventricle (DiV), above which lies the SCO. White rectangles in C and F delineate the close-up on cilia (C’, F’). Scale bar: 10 µm for A-F, 2.5 µm for C’-F’. (G–L) Immunostaining for Acetylated Tubulin (G, J), GFAP (H, K) and LCP1 (I, L) on transverse sections of the brain at SCO level in controls (G–I) (n=3), and cep290-/- (J–L) (n=3) juvenile (4 wpf) fish. In G and J, nuclei are stained with DAPI (blue). Scale bar: identical to A-F. (M) Graph representing the percentage of GFAP-positive cells among the total number of SCO secretory cells in control (n=3) and scoliotic cep290-/- (n=3) fish at scoliosis onset (N=1). Each dot represents the mean ratio of GFAP-positive over total SCO secretory cells in 5–7 sections per fish. (N) Quantification of LCP1 + cell density around the SCO in controls (n=3) and curved cep290-/- (n=3) fish at 4 wpf. 16 sections were counted for control, 18 sections for cep290-/- scoliotic fish. Each dot represents the mean value for one fish. Statistical analysis was performed with unpaired T test where * means p-value < 0.05. Error bars represent s.d. (O–V) Immunostaining for GFAP (white) on brain transverse sections at cerebellum levels (P, R) and anterior hindbrain level (T, V) in control (P, T) (n=3), and cep290-/- (R, V) (n=3) fish. Corresponding nuclear staining (DAPI in white) is shown above (O, Q, S, U). Scale bar: 125 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain (Danio rerio) | Zebrafish wild-type AB or (TL x AB) hybrid strains | IBPS aquatic facility, Paris | N/A | |
| Strain (Danio rerio) | Zebrafish rpgrip1l∆ mutant strain | This manuscript, Zfin: rpgrip1lbps1 | ZDB-ALT-240703–12 | |
| Strain (Danio rerio) | Zebrafish rpgrip1lex4 mutant strain | This manuscript, Zfin: rpgrip1lbps2 | ZDB-ALT-240703–13 | |
| Strain (Danio rerio) | Zebrafish urp2+/- | Gaillard et al., 2023; PMID:36736605 | ZDB-ALT-230207–5 | |
| Strain (Danio rerio) | Zebrafish dnaaf1tm317b/+ | van Rooijen et al., 2008; PMID:18385425 | ZDB-FISH-150901–27935 | |
| Strain (Danio rerio) | Zebrafish Scospondin-GFPut24 | Troutwine et al., 2020; PMID:32386529 | ZDB-FIG-200728–38 | |
| Antibody | anti-Bovine Reissner Fiber (rabbit polyclonal) | Didier et al., 1995; PMID:7577440 | Courtesy of Dr. S.Gobron | IF(1:200) |
| Antibody | anti-Human ZO1 (Mouse monoclonal) | Thermofisher | Cat#:33–9100 RRID:AB_2533147 | IF: (1:150) |
| Antibody | anti-human acetylated-tubulin (clone 6-11B-1) (Mouse monoclonal) | Sigma-Aldrich | Cat#:T 6793 RRID:AB_477585 | IF: (1:400) |
| Antibody | anti glutamylated Tubulin (GT335 clone, Mouse monoclonal) | Adipogen | Cat#:AG-20B-0020-C100 | IF: (1:500) |
| Antibody | anti-human Arl13b (Rabbit polyclonal) | Proteintech | Cat#:17711–1-AP | IF: (1:200) |
| Antibody | anti human GFAP (mouse monoclonal) | Sigma | Cat#:G3893 | IF: (1:400) |
| Antibody | Anti GFP (chicken monoclonal) | AVES lab | Cat#:GFP-1020 | IF: (1:200) |
| Antibody | Anti Myc (clone 9B-11, mouse monoclonal) | Cell signaling | Cat#:2276 | IF: (1:200) |
| Antibody | Anti zebrafish LCP1 (rabbit polyclonal) | GeneTex | Cat#:GTX124420 | IF: (1:200) |
| Antibody | anti-human gamma-tubulin (Clone GTU-88, mouse monoclonal) | Sigma | Cat#:T6557 | IF:(1:500) |
| Antibody | Anti-human annexin-2 (rabbit polyclonal) | Proteintech | Cat#:11256–1-AP RRID:AB_2057311 | IF: (1:200) |
| Antibody | Anti-mouse IgG1 Alexa633(goat polyclonal) | Molecular probes | Cat#:A-21126 RRID:AB_2535768 | IF: (1:400) |
| Antibody | Anti-mouse IgG1 Alexa568(goat polyclonal) | Molecular Probes | Cat#:A-21124 RRID:AB_2535766 | IF: (1:400) |
| Antibody | Anti-mouse IgG2a Alexa568(goat polyclonal) | Molecular probes | Cat#:A-21134 RRID:AB_2535773 | IF: (1:400) |
| Antibody | Anti-mouse IgG2a Alexa488(goat polyclonal) | Molecular probes | Cat#:A-21131 RRID:AB_141618 | IF: (1:400) |
| Antibody | Anti-mouse IgG2b Alexa633(goat polyclonal) | Molecular probes | Cat#:A-21146 RRID:AB_2535782 | IF: (1:400) |
| Antibody | Anti-mouse IgG2b Alexa568 (goat polyclonal) | Molecular probes | Cat#:A-21144 RRID:AB_2535780 | IF: (1:400) |
| Antibody | Anti-rabbit IgG Alexa568 (goat polyclonal) | Molecular probes | Cat#:A-11011 RRID:AB_2535780 | IF: (1:400) |
| Antibody | Anti-rabbit IgG Alexa488 (goat polyclonal) | Molecular probes | Cat#:A-11008 RRID:AB_143165 | IF: (1:400) |
| Antibody | Anti-Chicken IgY -FITC (donkey polyclonal) | Jackson Immuno-Research | Cat#:703-096-155 RRID:AB_2340357 | IF: (1:200) |
| Recombinant DNA reagent | foxj1a:: 5xMyc-RPGRIP1L (Tol2 construct) | This paper | Built by Gateway method, see Materials and methods, transgenesis section | |
| Recombinant DNA reagent | col2a1a:: 5xMyc-RPGRIP1L (Tol2 construct) | This paper | Built by Gateway method, see Materials and methods, transgenesis section | |
| Sequence-based reagent | Rpgrip1l_ex25F | This paper | Genotyping primers | AGTGTGCGGTACATCTCCAA |
| Sequence-based reagent | Rpgrip1l-ex4-del F | This paper | Genotyping primers | CCCACACTGCATACGCACTC |
| Sequence-based reagent | Rpgrip1l_ex25_R3 | This paper | Genotyping primers | GTTGTGTCTCTGCCATATATTG |
| Sequence-based reagent | Urotensin2 family neuro-peptides | Gaillard et al., 2023; PMID:36736605 | Q-PCR primers | Listed in Supplementary file 1 of Gaillard et al. |
| Sequence-based reagent | Rpgrip1l_x4_G1 | This paper | Cas9-Guide RNA | GCTTACGGTCCTTCACCAGACGG |
| Sequence-based reagent | Rpgrip1l_x25_G3 | This paper | Cas9-Guide RNA | CCTCAGTTGACAGGTTTCAGCGG |
| Commercial assay or kit | Gateway BP Clonase II Enzyme Mix | Invitrogen | Cat#:11789–020 | |
| Commercial assay or kit | Gateway LR Clonase II Plus Enzyme Mix | Invitrogen | Cat#:12538–120 | |
| Commercial assay or kit | Megascript T7 Transcription Kit | Thermo Fisher Scientific, Waltham, MA | Cat#:AM1334 | |
| Commercial assay or kit | miRNAeasy QIAGEN kit | QIAGEN | Cat#:217004 | |
| Commercial assay or kit | QIAEX II gel extraction kit | QIAGEN, | Cat#:20021 | |
| Commercial assay or kit | KAPA mRNA Hyperprep kit | Roche | Cat#:08098115702 | |
| Chemical compound, drug | NACET | Biolla Chemicals | Cat#:59587-09-6 | |
| Chemical compound, drug | Vectashield | Vector Laboratories | Cat#:H-1000 | |
| Recombinant DNA reagent | Foxj1 promoter-enhancer sequence | Grimes et al., 2016; PMID:27284198 | ||
| Recombinant DNA reagent | Col2a1a promter-enhancer | Dale and Topczewski, 2011; PMID:21723274 | ||
| Software | LASX | Leica | RRID:SCR_013673 | |
| Software | Amira for Life & Biomedical Sciences | Thermo Fisher Scientific | RRID:SCR_007353 | |
| Software | CRISPOR | Haeussler et al., 2016 | http://crispor.tefor.net/ | |
| Software | Fiji/ImageJ | Schindelin et al., 2012 | https://imagej.net/Fiji/Downloads | |
| Software | PRISM | GraphPad | RRID:SCR_002798 | https://www.graphpad.com/ |
| Software | Metascape | RRID:SCR_016620 | https://metascape.org/gp/index.html#/main/step1 | |
| Software | Matlab | The Mathworks, Inc. | RRID:SCR_001622 | https://fr.mathworks.com/products/matlab.html |
| Software | NRecon reconstruction software | Micro Photonics Inc. | https://www.microphotonics.com/micro-ct-systems/nrecon-reconstruction-software/ | |
| Software | CTvox | Bruker | https://www.microphotonics.com/micro-ct-systems/visualization-software/ | |
| Software | CT Analysis | Bruker | https://www.microphotonics.com/micro-ct-systems/ct-analysis/ | |
| Other | Refractometer | Roth Sochiel EURL DR 301–95 | Measure of refraction index of mounting medium used for IF on cleared brains (Materials and methods) | |
| Other | Confocal microscope | Leica (SP5) | Whole mount IF imaging on zebrafish embryos | |
| Other | Confocal microscope | Zeiss (LSM 980 Inverted) | Imaging IF of brain paraffin sections | |
| Other | Confocal microscope | Zeiss (LSM 710) | Imaging IF of brain paraffin sections | |
| Other | Macroscope | Zeiss (AXIOZOOM V16) | Lcp1 positive cells quantification on whole brain | |
| Other | Micro-scanner | Bruker (Skyscan 1272) | See µCT scans in Materials and methods | |
| Other | Microtome | Leica (RM2125RT) | See Paraffin sectioning of brains for IF (Materials and methods) | |
| Other | Q-PCR apparatus | Applied Biosystems (Step One plus) | Q-PCR analysis (Materials and methods) |
Additional files
-
Supplementary file 1
Excel table providing the list of differentially expressed genes within the dorsal trunk of rpgrip1l-/- fish versus controls at 5 wpf.
- https://cdn.elifesciences.org/articles/96831/elife-96831-supp1-v1.xlsx
-
Supplementary file 2
Excel table providing the list of differentially expressed genes within the brain of rpgrip1l-/- fish versus controls at 5 wpf.
- https://cdn.elifesciences.org/articles/96831/elife-96831-supp2-v1.xlsx
-
Supplementary file 3
List of the Foxj1 direct (first tab) and indirect (second tab) target genes upregulated in the rpgrip1l-/- transcriptomes compared to controls.
- https://cdn.elifesciences.org/articles/96831/elife-96831-supp3-v1.xlsx
-
Supplementary file 4
Excel file providing the list of deregulated proteins in the rpgrip1l-/- proteome compared to controls.
- https://cdn.elifesciences.org/articles/96831/elife-96831-supp4-v1.xlsx
-
Supplementary file 5
Graphical abstract.
- https://cdn.elifesciences.org/articles/96831/elife-96831-supp5-v1.tiff
-
MDAR checklist
- https://cdn.elifesciences.org/articles/96831/elife-96831-mdarchecklist1-v1.docx





