The actomyosin system is essential for the integrity of the endosomal system in bloodstream form Trypanosoma brucei
Figures
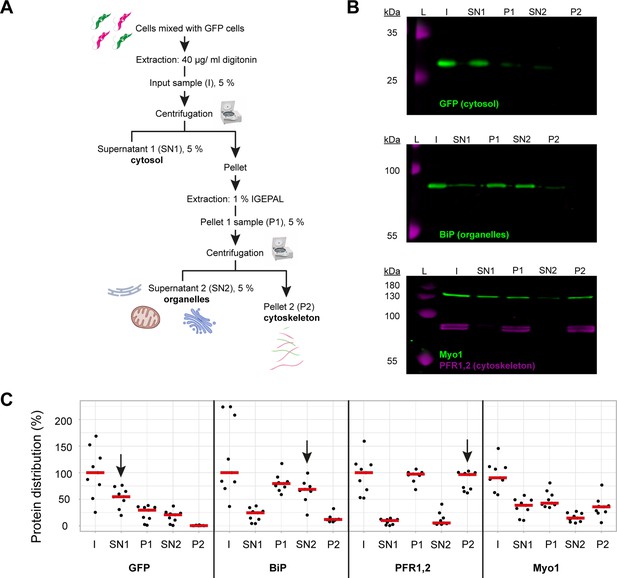
TbMyo1 has a large cytosolic pool.
(A) Schematic representation of the two-step fractionation protocol. Bloodstream form (BSF) T. brucei cells were mixed with GFP-expressing BSF cells and extracted with digitonin to release the cytosolic fraction. The permeabilised cells were subsequently extracted with IGEPAL (non-ionic detergent) to release the organelle fraction. 5% samples of the input (I), supernatant (SN1, SN2) and pellet (P1, P2) fractions were taken at the indicated points. The samples were separated by SDS-PAGE and analysed by immunoblotting. (B) TbMyo1 is present in both a cytosolic and a cytoskeleton-associated pool. Exemplary immunoblotting results from the two-step fractionation protocol as described in (A). Equal fractions (~2%) were loaded in each lane. The cytosolic marker (GFP, ~27 kDa) was mainly detected in the SN1 fraction. The ER chaperone BiP (~80 kDa) partitioned into the P1 and then into the SN2 fractions. The flagellar cytoskeleton proteins PFR1,2 (~79 + 82 kDa) partitioned into the P1 and then the P2 fractions. TbMyo1 (~130 kDa) was found to be nearly equally divided between the SN1 and P1 fractions. The P1 fraction almost completely partitioned into the P2 fraction. Exemplary images from multiple (n = 8) independent experiments are shown. L, molecular weight ladder. (C) Nearly 50% of TbMyo1 is cytosolic. Quantification of the fractionation immunoblot data (n = 8). The red bars indicate the median.
-
Figure 1—source data 1
PDF file containing original immunoblots for Figure 1B indicating the relevant areas; spreadsheet with raw data for panel C.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-data1-v1.zip
-
Figure 1—source data 2
Original files for panel images displayed in Figure 1B.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-data2-v1.zip
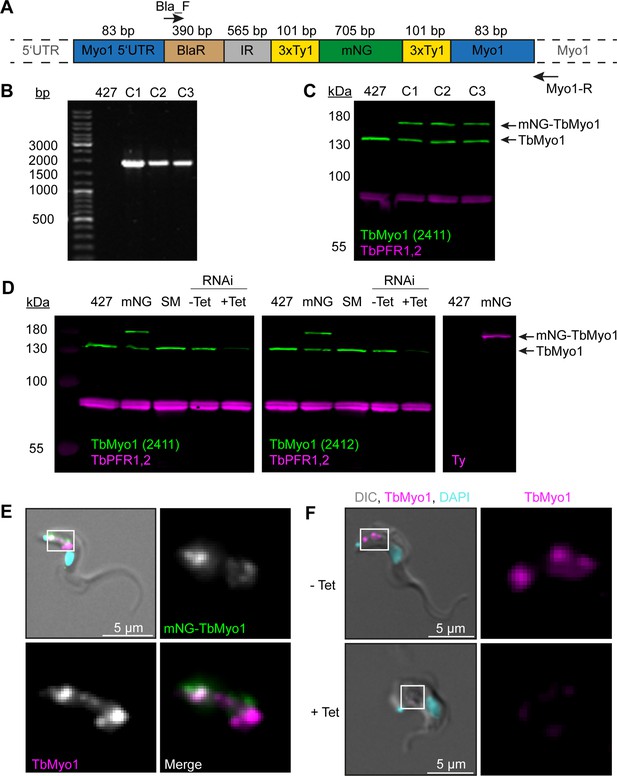
Generation and validation of TbMyo1 antibodies and cell lines.
(A) Schematic of the TbMyo1 in situ tagging construct integrated into the genome. The construct encoded the 3' end of the TbMYO1 5’ UTR, a blasticidin (BlaR) resistance gene, the alpha/beta tubulin intergenic region (IR), the mNG gene flanked by two 3xTy1 epitope tags, and the 5’ end of the TbMYO1 ORF. The sizes of the various elements are shown in the schematic. The 83 bp labels indicate the homology arms that drive homologous recombination at the MYO1 locus. The annealing sites of the two primers used to confirm integration at the endogenous MYO1 locus (Bla-F, Myo1-R) are indicated with arrows. Note that the schematic elements are not shown to scale. (B) Correct integration of the TbMyo1 tagging construct at the endogenous locus. The gDNA of wild-type (427) and three candidate mNG-TbMyo1 clones (C1–C3) was purified and analysed by PCR. The annealing sites of the PCR primers are shown in (A) above. No product was observed for the wild-type control. An ~1700 bp product was obtained from the three mNG-TbMyo1 candidates, indicating correct integration. (C) Expression of mNG-TbMyo1. Whole-cell lysates from the three mNG-TbMyo1 clones and a wild-type control were prepared and analysed by immunoblotting. Anti-TbMyo1(2411) was used as a primary antibody; anti-TbPFR1,2 (L13D6) antibodies were used to detect the housekeeping proteins PFR1,2. The endogenous TbMyo1 signal (green) was visible in all lanes at ~130 kDa; C1, C2, and C3 additionally expressed an ~170 kDa protein, matching the size of the mNG-TbMyo1. (D) Validation of anti-TbMyo1 specificity. Whole-cell lysates from four different cell lines (wild-type (427), single marker (SM), mNG-TbMyo1 (mNG), TbMyo1 RNAi) were immunoblotted with two anti-TbMyo1 antisera (2411, 2412). The TbMyo1 RNAi lysates were obtained from uninduced (-Tet) and tetracycline-induced (+Tet) samples. TbPFR1,2 was used as a loading control. Both antisera were able to detect the endogenous TbMyo1 protein at ~130 kDa. The signal intensities of the 427 cells and the single marker (SM) ones were almost equal, independent of which antiserum was used. An additional band at ~170 kDa was observed in the mNG-TbMyo1 samples. This upper band could also be detected using anti-Ty1 antibodies (right-hand image). In the RNAi-induced (+Tet) cells, the signal from both antisera was weaker. (E) Strong overlap between endogenous and mNG-tagged TbMyo1. The mNG-TbMyo1 clones were fixed, labelled with anti-TbMyo1 antibodies (magenta), and imaged using widefield microscopy. DNA (cyan) was stained using DAPI. Both the mNG-TbMyo1 (green) and the endogenous protein were exclusively localised to the posterior region of the cells. Identical results were obtained using anti-Ty1 antibodies to label the mNG-TbMyo1. The same pattern was observed in all three clones; an exemplary image is shown. (F) Depletion of TbMyo1 results in a loss of signal and morphological changes. TbMyo1 RNAi cells were induced for 48 hr, fixed, and labelled with anti-TbMyo1 antibodies (magenta). DNA was stained with DAPI (cyan). Control cells showed an intense signal of TbMyo1 (magenta) between the nucleus and the kinetoplast. Induced cells showed a nearly complete loss of the signal, with a small degree of clone-to-clone variation. The morphology of the TbMyo1-depleted cells changed dramatically, with some cells developing a ‘Big Eye’ phenotype. The same results were obtained with three separate clones; an exemplary image is shown.
-
Figure 1—figure supplement 1—source data 1
PDF file containing original gels and immunoblots for Figure 1—figure supplement 1B, C, D, indicating the relevant areas.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Original files for panel images displayed in Figure 1—figure supplement 1B, C, D.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-figsupp1-data2-v1.zip
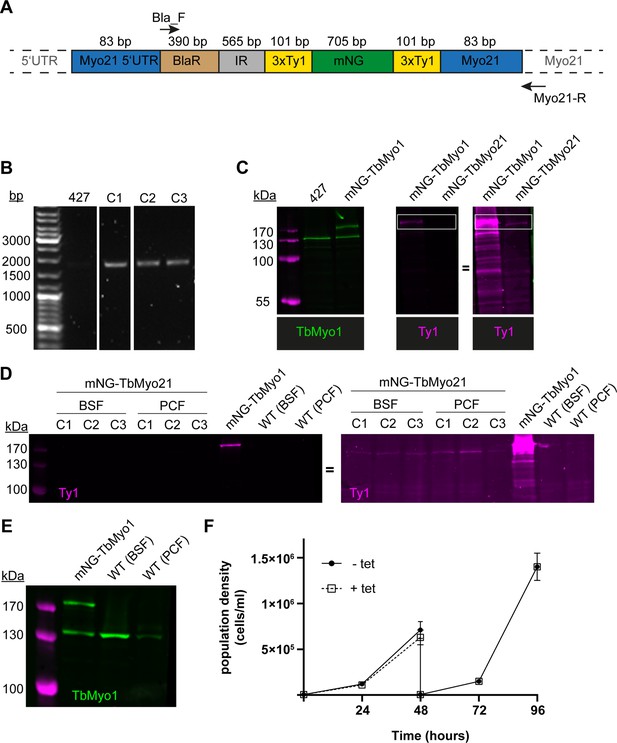
TbMyo21 is expressed at an extremely low level.
(A) Generation of mNG-tagged TbMyo21 cell line. Schematic of the TbMyo21 in situ tagging construct integrated into the genome. The construct encoded the 3' end of the TbMYO21 5’ UTR, a blasticidin (BlaR) resistance gene, the alpha/beta tubulin intergenic region (IR), the mNG gene flanked by two 3xTy1 epitope tags, and the 5’ end of the TbMYO21 ORF. The sizes of the various elements are shown in the schematic. The 83 bp labels indicate the homology arms that drive homologous recombination at the MYO21 locus. The annealing sites of the primers used to confirm integration at the endogenous MYO21 locus (Bla-F, Myo21-R) are indicated with arrows. Note that the schematic elements are not shown to scale. (B) Correct integration of the TbMyo21 tagging construct at the endogenous locus. The gDNA of wild-type (427) and three candidate mNG-TbMyo1 clones (C1–C3) was purified and analysed by PCR. The annealing sites of the PCR primers are shown in panel A. No product was observed for the wild-type control (427). An ~2000 bp product was obtained from the three mNG-TbMyo21 candidates, indicating correct integration. (C) mNG-TbMyo21 is expressed at a very low level relative to TbMyo1. Whole-cell lysates from a wild-type control, the mNG-TbMyo1 cell line, and the mNG-TbMyo21 cell line were prepared and analysed by immunoblotting. The endogenous TbMyo1 signal (green) was visible at ~130 kDa; the mNG-TbMyo1 cell line additionally expressed an ~170 kDa protein, corresponding to mNG-TbMyo1. The anti-Ty1 antibody signal (magenta) required an overexposure (right hand image) to visualise the mNG-TbMyo21 band at ~160 kDa (boxed area). (D) TbMyo21 expression levels are equally low in bloodstream form (BSF) and procyclic form (PCF) cells. Whole-cell lysates from the mNG-TbMyo21 cell line and a wild-type control were prepared before and after differentiation to PCF cells and analysed by immunoblotting. A whole-cell lysate from BSF mNG-TbMyo1 cells served as a positive control. The anti-Ty1 antibody signal (magenta) was only visible in the mNG-TbMyo1 cell line at ~170 kDa (left-hand image). The anti-Ty1 signal required an overexposure to visualise an mNG-TbMyo21 band at ~160 kDa (right-hand image). (E) TbMyo1 expression is downregulated after differentiation to PCF cells. The whole-cell lysates from BSF mNG-TbMyo1 cells and both BSF and PCF wild-type cells were analysed by immunoblotting. Equal numbers of cell equivalents (1.4 × 106) were loaded in each lane. The endogenous TbMyo1 signal (green) was visible in all lanes at ~130 kDa; the mNG-TbMyo1 cell line additionally expressed an ~170 kDa protein, matching the size of the mNG-TbMyo1. Quantification was by direct measurement of band intensity after background subtraction and without normalisation to a loading control, and is therefore somewhat tentative. The TbMyo1 signal in PCF cells showed <50% signal intensity compared to BSF cells. (F) TbMyo21 does not appear to be essential for growth in vitro. The cell population density of TbMyo21 RNAi cells was measured for 96 hr in tetracycline uninduced (-Tet) and induced (+Tet) conditions. The cells were diluted after 48 hr to ensure logarithmical growth. Results from three independent experiments using three separate clones are shown; data points are mean ± standard deviation.
-
Figure 1—figure supplement 2—source data 1
PDF file containing original gels and immunoblots for Figure 1—figure supplement 2B, C, D,E indicating the relevant areas; spreadsheet with raw data for Figure 1—figure supplement 2F.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-figsupp2-data1-v1.zip
-
Figure 1—figure supplement 2—source data 2
Original files for panel images displayed in Figure 1—figure supplement 2B, C, D,E.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig1-figsupp2-data2-v1.zip
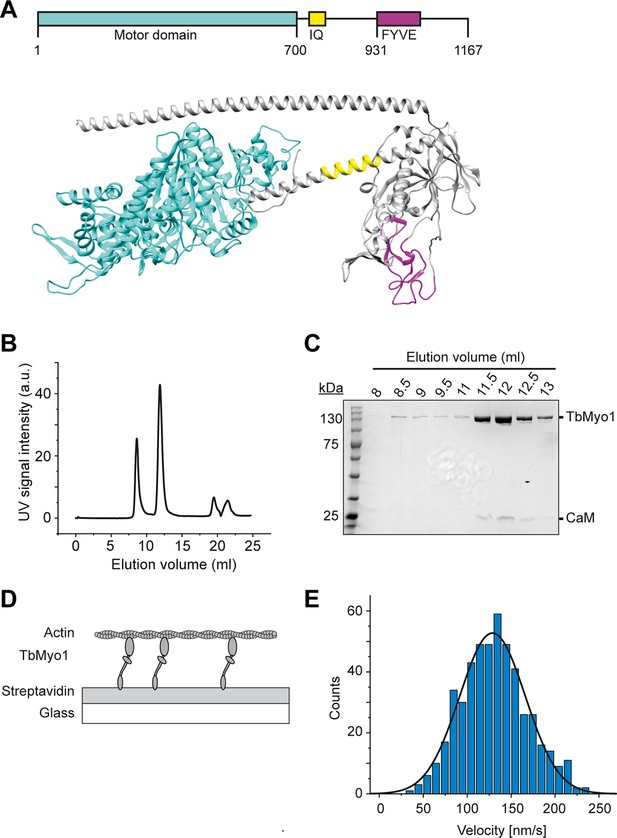
TbMyo1 translocates filamentous actin in vitro.
(A) Schematic illustration and structural prediction of the domain architecture of TbMyo1. The motor domain is shown in cyan, the IQ (calmodulin-binding) motif is shown in yellow, and the FYVE domain is shown in magenta. Regions without domain prediction are displayed in grey. The structural prediction was generated using AlphaFold (ID: AF-Q585L2-F1). (B) Size-exclusion profile of affinity-purified TbMyo1 (Superdex 200 Increase 10/300). The monomeric TbMyo1 fraction eluted in a single peak at around 12 ml. The 9 ml (void volume) elution peak corresponds to aggregated protein. (C) TbMyo1 eluted mainly in the second protein peak. Samples were taken from the eluted volumes corresponding to the different protein peaks and analysed by SDS-PAGE. TbMyo1 (130 kDa) eluted together with human calmodulin in the 11.5–12.5 ml elution fractions. (D) Schematic representation of the in vitro actin filament gliding assay. Monomeric, C-terminally biotinylated TbMyo1 was immobilised via streptavidin on a glass surface. TbMyo1 translocated Alexa488-phalloidin labelled filamentous actin (F-actin) in the presence of ATP. (E) TbMyo1 translocates filamentous actin in vitro. Measurements were taken at 22°C with a final concentration of 2 mM ATP (n = 30 actin filaments). The average velocity was 130 ± 40 nm/s (mean ± SD; Gaussian fit).
-
Figure 2—source data 1
Spreadsheet with raw data for Figure 2E.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig2-data1-v1.zip
TbMyo1 translocate filamentous actin in vitro.
Rhodamine-phalloidin-stained actin filaments gliding over a coverslip decorated with full-length TbMyo1 molecules. Buffer contained 2 mM ATP. Images were acquired at a rate of 1 frame/s. Playback rate 50 frames/s. Scale bar 5 μm.
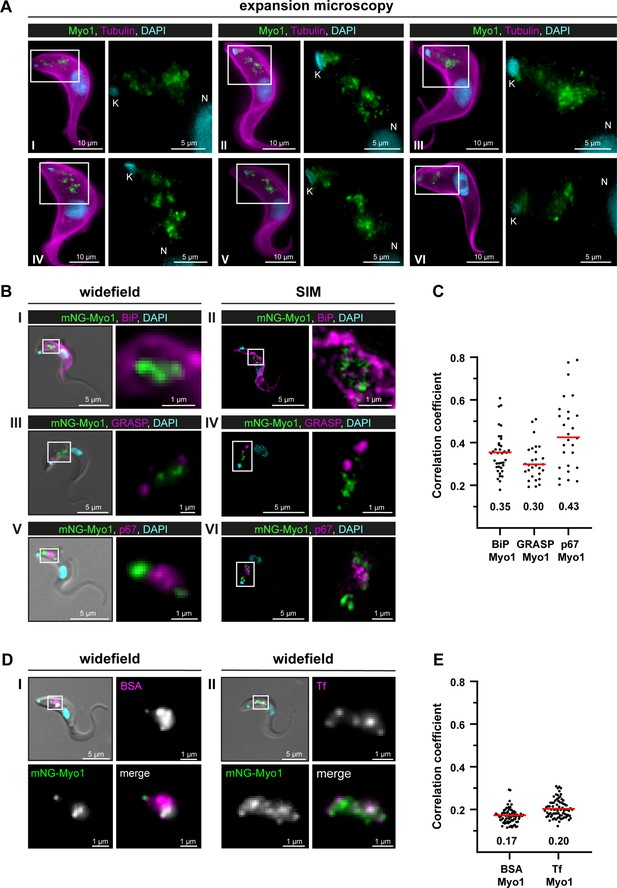
TbMyo1 is associated with the endocytic and not the biosynthetic pathway.
(A) TbMyo1 is concentrated on structures in the posterior region of the cell. Expansion microscopy of bloodstream form (BSF) T. brucei labelled with anti-tubulin (yellow), anti-TbMyo1 (magenta), and DAPI (cyan). Six exemplary cells are shown (I–VI). For all cells, a magnified image of the posterior region (boxed area) is shown without the tubulin signal. The TbMyo1 signal was visible as a large cluster of foci in the posterior region between nucleus (N) and kinetoplast (K). (B) TbMyo1 does not colocalise with the ER (BiP, panels I and II), Golgi apparatus (GRASP, panels III and IV), or lysosome (p67, panels V and VI) markers. Fixed BSF cells were labelled using the indicated antibodies or tags and imaged using widefield microscopy and structured illumination microscopy (SIM). Overlay images with DIC and DAPI are shown for widefield microscopy images; SIM images show a merge of all three fluorescence channels. Magnified images of the regions of interest for TbMyo1 and the indicated markers are shown. (C) Quantification of correlation between TbMyo1 and organelle marker signals. Correlation was estimated using Spearman’s rank correlation (BiP/TbMyo1 n = 38, GRASP/TbMyo1 n = 28, p67/TbMyo1 n = 27). The median ρ is represented by a red line and the corresponding number is written below the data plot. (D) TbMyo1 overlaps with endocytic cargo. Cells expressing mNG-TbMyo1 were incubated with fluorescent cargo, fixed, and imaged using widefield microscopy. TbMyo1 (green) and the cargo markers (magenta) showed partial (BSA, panel I) or strong (transferrin, Tf, panel II) overlap. A magnified image of the fluorescence channels and a merge are shown next to the overlay with DIC. (E) Quantification of correlation between TbMyo1 and endocytic cargo marker signals. Correlation was estimated using Spearman’s rank correlation (BSA/TbMyo1 n = 69, Tf/TbMyo1 n = 77). The median ρ is represented by a red line and the corresponding number is written below the data plot. Exemplary images from multiple (n > 2) independent experiments are shown.
-
Figure 3—source data 1
Spreadsheets with raw data for Figure 3C and E.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig3-data1-v1.zip
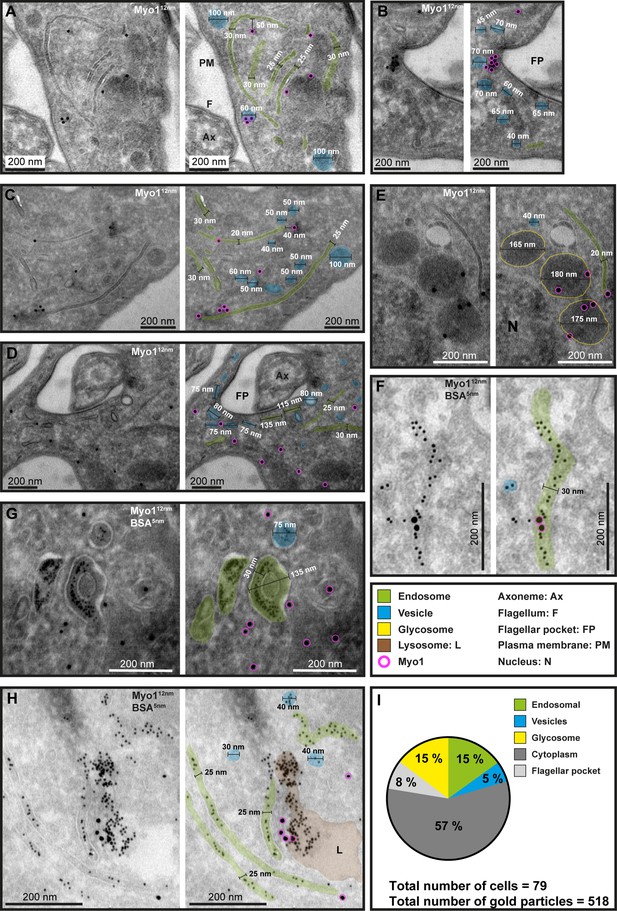
Ultrastructural localisation of TbMyo1.
(A–H) TbMyo1 localises to endosomal tubes, vesicles, glycosomes, the flagellar pocket, and the cytoplasm. Electron micrographs of cryosections labelled with anti-TbMyo1 and 12 nm gold-conjugated secondary antibodies. For each panel, a raw and a pseudocoloured version of the image are presented. Endosomal tubes are coloured green. Vesicles are coloured blue. Glycosomes are outlined in yellow. The lysosome is coloured brown. Gold particles corresponding to TbMyo1 signals are outlined in magenta. (F–H) Cells were incubated with BSA conjugated to 5 nm gold particles prior to fixation and immunolabelling with anti-TbMyo1. The BSA was observed in vesicles, endosomes, and the lysosome. (I) Distribution of TbMyo1 in the cytoplasm and on subcellular structures. All gold particles 30 nm or closer to a structure were defined as being on the structure (based on a conservative calculation that two antibodies, each 15 nm in length, are between the bound epitope and the gold particle). More than half of the gold particles were found to be cytoplasmic. The total number of cells and gold particles quantified is indicated. Abbreviations: axoneme (Ax), flagellum (F), flagellar pocket (FP), plasma membrane (PM), and nucleus (N). Exemplary single- and double-labelled images were chosen from three separate labelling experiments.
-
Figure 4—source data 1
Spreadsheet with raw data for Figure 4I.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig4-data1-v1.zip
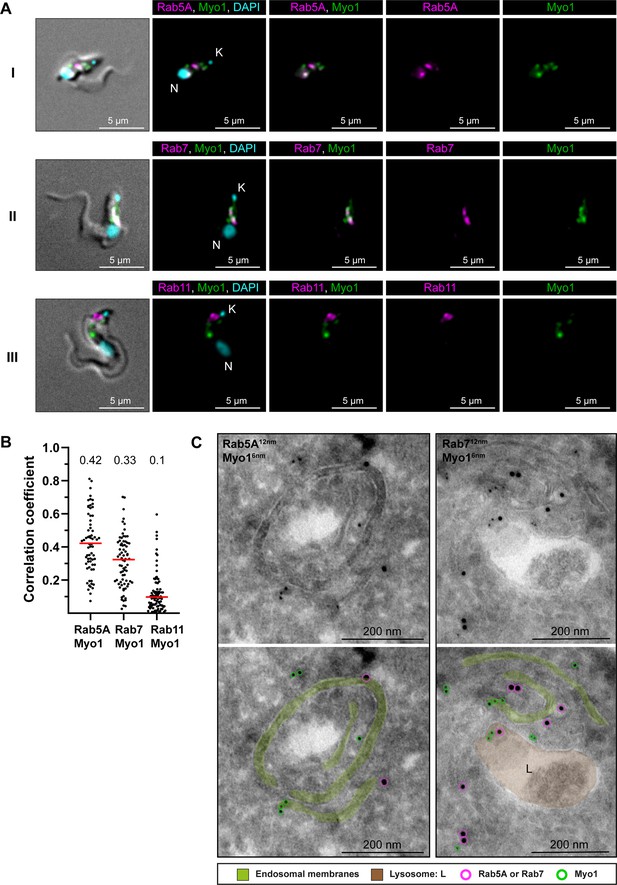
TbMyo1 is associated with early and late endosomes.
(A) TbMyo1 signals partially overlap with TbRab5A and TbRab7 signals, but not with TbRab11 signals. Colocalisation experiments were conducted using chemically fixed mNG-TbMyo1 cells, labelled with antibodies against TbRab5A, 7, or 11 and imaged using widefield microscopy. The fluorescence signals for TbRab5A (I, magenta), TbRab7 (II, magenta), TbRab11 (III, magenta) and TbMyo1 (I–III, green) were localised between the kinetoplast (K) and the nucleus (N) of the cells. DNA was stained using DAPI (I–III, cyan). Exemplary cells from four independent experiments are shown. (B) Quantification of correlation between TbMyo1 and TbRab signals. Correlation was estimated using Pearson’s correlation coefficient (TbRab5A/TbMyo1, n = 66; TbRab7/TbMyo1, n = 73; TbRab11/TbMyo1, n = 86). Red bars indicate the median R; median R values for each pair are shown above the dot plot. (C) Electron micrographs of cryosections colabelled with anti-TbMyo1 and anti-TbRab5A or anti-TbRab7 antibodies. For each panel, a raw and a pseudocoloured version of the image are presented. Endosomal membranes are coloured green. The lysosome is coloured brown. Gold particles corresponding to TbMyo1 signals are outlined in green. Gold particles corresponding to TbRab5A or TbRab7 signals are outlined in magenta. Exemplary cells from a single labelling experiment are shown.
-
Figure 4—figure supplement 1—source data 1
Spreadsheet with raw data for Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig4-figsupp1-data1-v1.zip
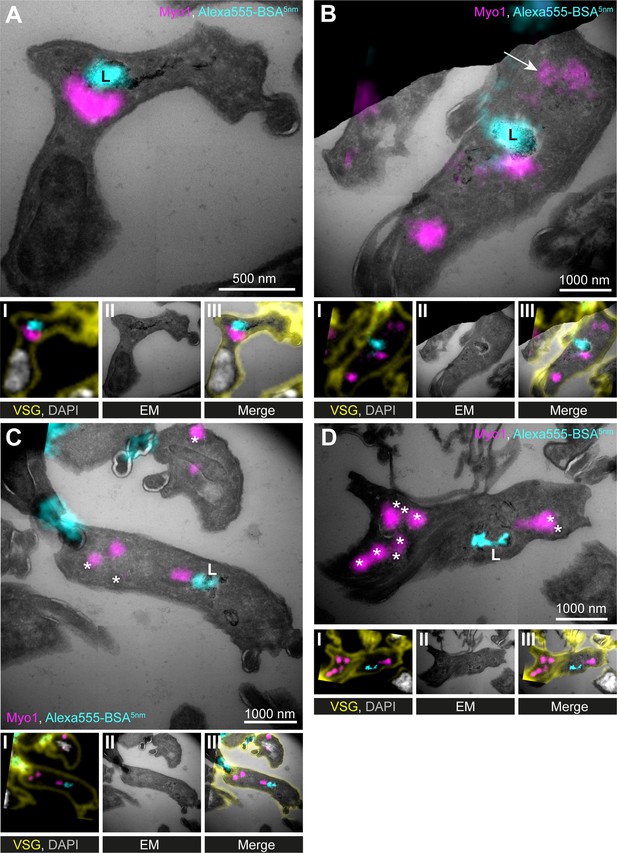
TbMyo1 is clustered adjacent to the lysosome.
(A–D) Correlative light and electron microscopy (CLEM) imaging of cryosections. To visualise cargo trafficking, cells were incubated with double-labelled Alexa555- and 5 nm gold-conjugated BSA (cyan) before fixation. Cryosections were labelled with anti-TbMyo1 (magenta), anti-VSG (yellow), and DAPI (grey). For each panel, an image of the widefield microscopy (I), the electron micrograph (II) and an overlay of both images (III) is presented. The electron micrographs are manually stitched mosaics composed of multiple (4–9) tiled images of higher magnification (15,000–25,000×). Note that a fluorescence signal could only be detected at high local concentrations of Alexa555-BSA-5 nm gold. VSG and DAPI signals were used as fiducials to correlate IF and EM images. Correlations were made using the ec-CLEM plug-in in Icy for the initial correlation and Adobe Photoshop for the final overlay. Abbreviations: L, lysosome; *glycosomes; the arrow in (B) shows endosomal tubes filled with BSA gold and positive for TbMyo1. Images show exemplary images from a sample of 17 correlated cells.
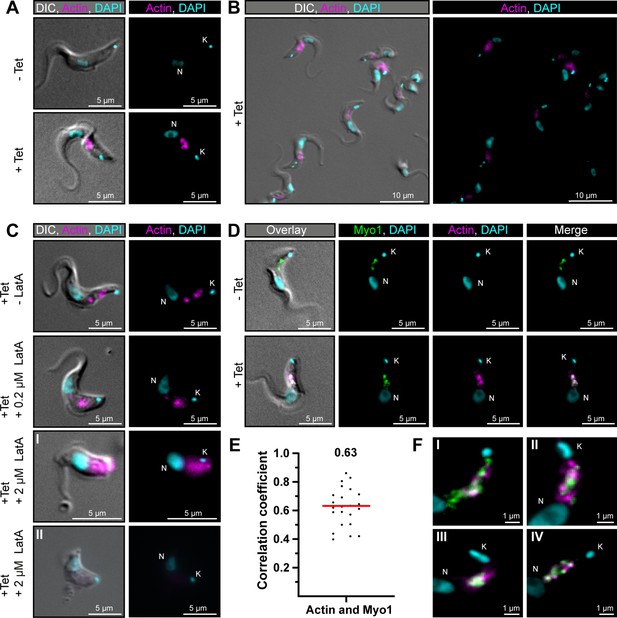
TbMyo1 overlaps extensively but not completely with actin.
(A) Imaging of trypanosome actin using an inducibly expressed anti-actin chromobody. Tetracycline (Tet) was used to induce expression of the anti-actin chromobody. Widefield microscopy of fixed cells in the absence (- Tet) or presence (+Tet) of tetracycline are shown. The induced (+Tet) cells showed a chromobody signal (magenta) in the posterior region of the cell between the nucleus (N) and kinetoplast (K), consistent with the reported localisation of actin in BSF T. brucei. DNA was stained with DAPI. (B) Cell-to-cell variation of the chromobody signal in the tetracycline-induced population. The intensity but not the distribution of the signal varied from cell to cell. (C) The actin depolymerising drug latrunculin A (LatA) disrupts the chromobody signal. Widefield microscopy of fixed, induced chromobody cells in the presence of different LatA concentrations. The cells in the absence of LatA (- LatA) acted as controls. The addition of 0.2 μM LatA led to a slight blurring of the chromobody signal (magenta), while 2 μM LatA resulted in strong blurring (I) or even loss of signal (II) accompanied by a disruption of cell morphology. (D) TbMyo1 strongly overlaps with the anti-actin chromobody signal. Non-induced cells acted as a control and showed no chromobody signal, with only a TbMyo1 signal (green). Induced cells displayed both a chromobody (magenta) and a TbMyo1 signal, which strongly overlapped in the posterior region of the cell. (E) Quantification of correlation between TbMyo1 and actin signals. Correlation was estimated using Spearman’s rank correlation (n = 23). The median ρ is represented by a red line and the corresponding number is written above the data plot. (F) Enlarged view of the strong overlap between TbMyo1 and anti-actin chromobodies in the posterior region of the cell. Magnified images of the posterior region of four different cells (I–IV) are shown. TbMyo1 and the anti-actin chromobodies strongly overlapped in all cells examined, but the number and intensity of discrete spots varied.
-
Figure 6—source data 1
Spreadsheet with raw data for Figure 6E.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig6-data1-v1.zip
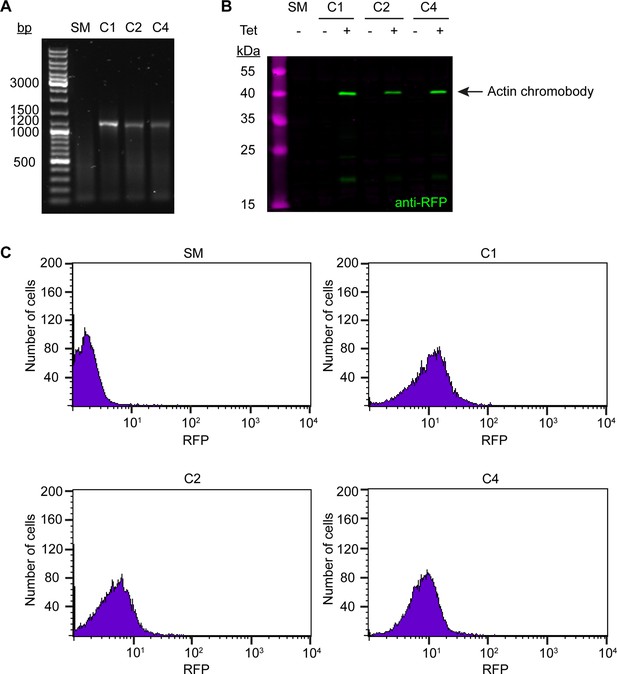
Generation and validation of the anti-actin chromobody cell line.
(A) Integration of the anti-actin TagRFP chromobody construct. The gDNA of wild-type (SM) and three candidate clones (C1, C2, C4) was purified and analysed by PCR using primers specific for the chromobody. No product was observed for the wild-type control. An ~1200 bp product was obtained from the three candidates, indicating integration into the genome. (B) Tight and inducible expression of the anti-actin TagRFP chromobody. Whole-cell lysates from the three clones (tetracycline-induced and -uninduced) and a wild-type control (SM) were prepared and analysed by immunoblotting, using anti-RFP antibodies. No signal was detected in the control sample and in the uninduced (-) chromobody clones. In the induced (+) samples, a protein band at ~40 kDa was detected in all three clones. The expected size of the anti-actin chromobody is 41.5 kDa. (C) Varying anti-actin TagRFP chromobody expression levels in the three clones. The three clones (C1, C2, C4) were analysed 24 hr after induction with tetracycline and RFP signal was measured using flow cytometry. Wild-type cells (SM) served as a control. All clones showed a significant increase in fluorescence counts in the RFP channel relative to control cells, with some clone-to-clone variation in expression levels.
-
Figure 6—figure supplement 1—source data 1
PDF file containing original gels and immunoblot for Figure 6—figure supplement 1A and B indicating the display areas.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig6-figsupp1-data1-v1.zip
-
Figure 6—figure supplement 1—source data 2
Original files for panel images displayed in Figure 6—figure supplement 1A, B, and C.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig6-figsupp1-data2-v1.zip
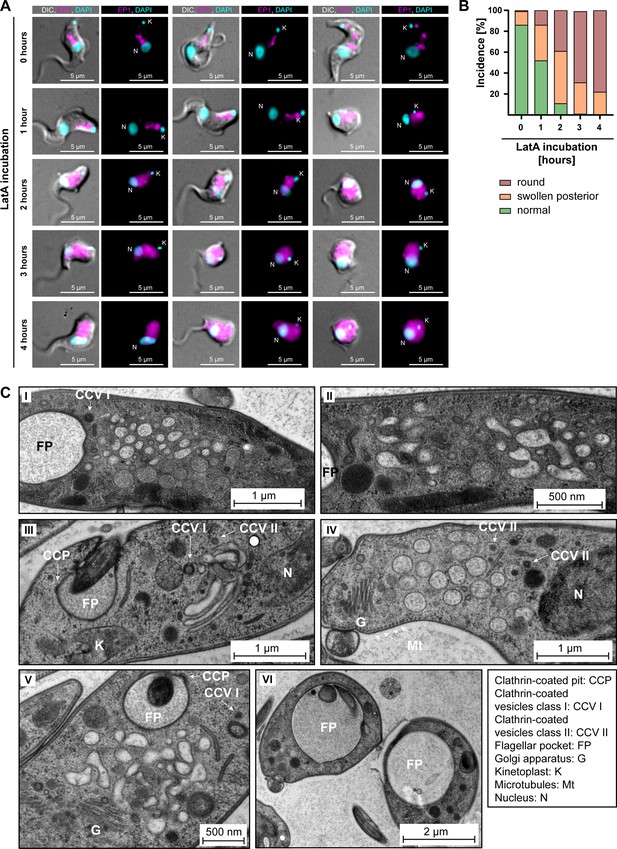
Actin depolymerisation disrupts the endosomal system.
(A) Latrunculin A (LatA) treatment perturbs the endosomal system. EP1-GFP-expressing cells were incubated with 2 μM LatA for the indicated time periods, then fixed and imaged using widefield microscopy. DNA was stained using DAPI. Longer incubation with LatA resulted in aberrant cell morphologies and an increasingly diffuse EP1-GFP signal. Exemplary cells from a single experiment are shown. (B) Quantification of the morphological effects of LatA treatment. Cells were manually classified as displaying normal, swollen posterior, or rounded morphology. The degree of morphological disruption was proportional to the duration of incubation with LatA. Data acquired from >200 cells assayed in three independent experiments. (C) LatA treatment disrupts the ultrastructural morphology of the endosomal system. Cells expressing EP1-GFP were subjected to a 30–60 min incubation with 2 μM LatA, followed by high-pressure freezing, embedding in Epon, and examination through transmission electron microscopy. The cytoplasm contained many large and interconnected tubular profiles, consistent with a swollen endosomal system. An enlargement of the flagellar pocket was additionally observed. Exemplary cells from a single experiment are shown.
-
Figure 7—source data 1
Spreadsheet with raw data for Figure 7B.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig7-data1-v1.zip
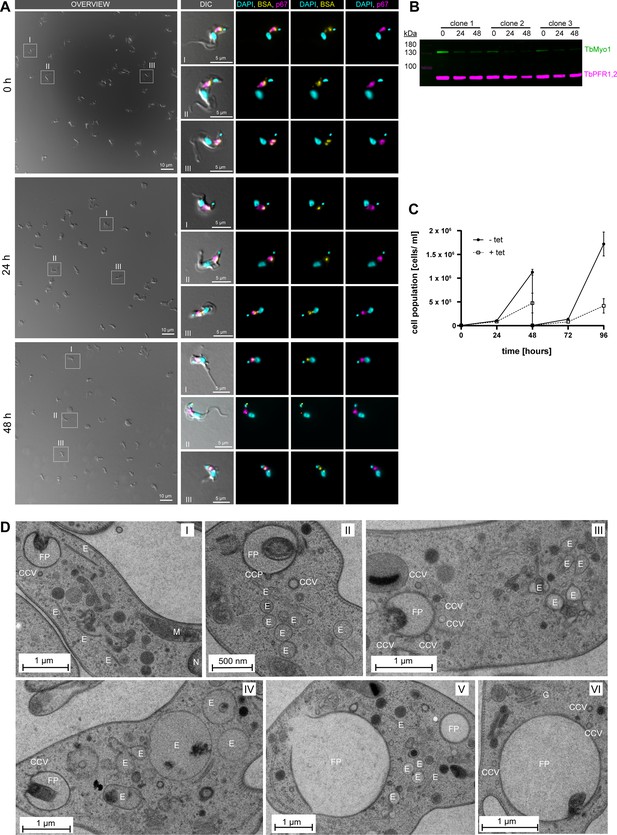
TbMyo1 depletion affects cell morphology and endosomal membrane organisation.
(A) Bovine serum albumin (BSA) is still internalised after TbMyo1 depletion. TbMyo1 depletion was induced by RNAi for 0, 24, and 48 hr. The cells were then harvested and incubated with fluorophore-conjugated BSA (yellow) for 30 min at 37°C. The cells were then fixed with formaldehyde and immunolabelled with anti-p67 antibodies (magenta), and DNA was stained with DAPI (cyan). Exemplary DIC fields of view are presented alongside enlarged views of selected morphologically normal cells (I–III). Prolonged protein depletion led to an increased number of cells displaying a rounded morphology. In cells with normal morphology, fluorescent BSA strongly overlapped with p67, indicating normal cargo uptake. Results obtained in a single experiment using three separate TbMyo1 RNAi clones. (B) Confirmation of TbMyo1 depletion. Whole-cell lysates from three different TbMyo1 RNAi clones were collected at 0, 24, and 48 hr and analysed by immunoblotting. The TbMyo1 signal (green) was observed at ~130 kDa, with signal intensity significantly decreasing at 24 hr and 48 hr in all three clones. Antibodies against TbPFR1,2 were used as loading controls to check for equal sample loading. (C) TbMyo1 is essential for in vitro growth. The cell population density of TbMyo1 RNAi cells was measured over 96 hr under control (-tet) and induced (+tet) conditions. Cells were diluted after 48 hr to maintain logarithmic growth. Results from two independent experiments, each using three separate clones, are shown; data points represent the mean ± standard deviation. (D) TbMyo1 depletion results in ultrastructural changes in endosomal membranes. BSF TbMyo1 RNAi cells were incubated for 24 hr to induce TbMyo1 depletion, followed by high-pressure freezing, embedding in Epon, and examination via transmission electron microscopy. Most cells exhibited either a normal ultrastructure (I) or an extremely enlarged flagellar pocket (V and VI). A subset of cells displayed numerous large and interconnected tubular profiles, suggestive of a swollen endosomal system. Exemplary cells from a single experiment are shown. Abbreviations: CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; E, endosomal membranes; FP, flagellar pocket; G, Golgi apparatus; M, mitochondrion; N, nucleus.
-
Figure 7—figure supplement 1—source data 1
PDF file containing original gels and immunoblots for Figure 7—figure supplement 1B, C, D, indicating the relevant areas.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig7-figsupp1-data1-v1.zip
-
Figure 7—figure supplement 1—source data 2
Original files for panel images displayed in Figure 7—figure supplement 1B, C, D.
- https://cdn.elifesciences.org/articles/96953/elife-96953-fig7-figsupp1-data2-v1.zip
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Lama pacos) | pAC-TagRFP | ChromoTek (Proteintech) | acr | See ‘Materials and methods’ for a description of subcloning |
| Antibody | Rabbit anti-TbMyo1(729–1168) polyclonal | This paper | 2411, 2412 | 1:5000-1:10,000 for immunoblotting; 1:500-1:2000 for immunofluorescence |
| Commercial assay or kit | REVERT Total Protein Stain | LI-COR | 926-11011 | Wash and Destaining solutions made in-house |
| Chemical compound, drug | Digitonin, ultra-pure | Merck | CAS 11024-24-1 | See ‘Materials and methods’ |
| Chemical compound, drug | Latrunculin A | Sigma | CAS 76343-93-6 | See ‘Materials and methods’ |





