Dysregulated Ca2+ signaling, fluid secretion, and mitochondrial function in a mouse model of early Sjögren’s disease
Figures
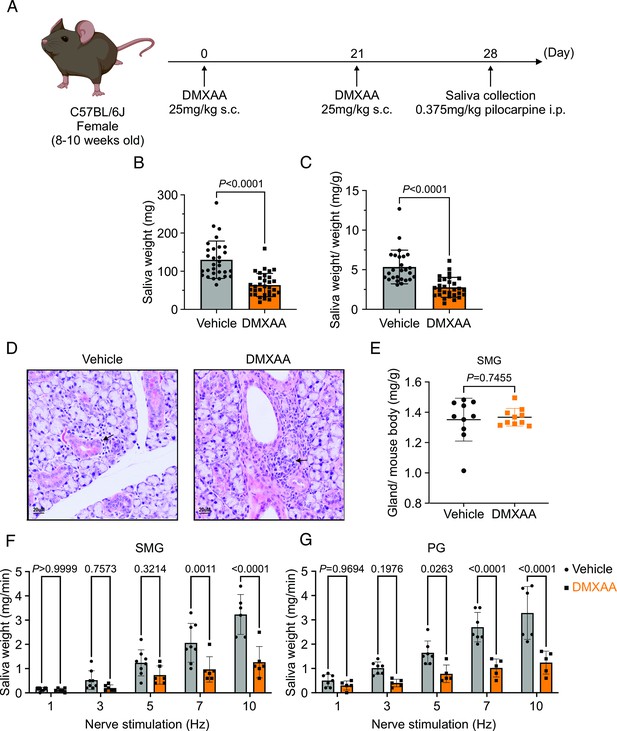
Deficiency in salivary secretion in 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-induced Sjögren's syndrome (SS) mouse model.
(A) Schematic timeline for the generation of the SS mouse model. Female wild-type (WT) mice were administered two subcutaneous doses of DMXAA on Day 0 and Day 21. Salivary gland function was assessed on day 28. (B–C) Saliva, stimulated by pilocarpine, was collected over 15 min. (B) The amount of saliva secretion was determined by measuring the saliva weight. Vehicle: n=30 mice, SS mouse model: n=32 mice. Mean ± SD. (C) The weight of collected saliva was normalized to each mouse’s body weight. Vehicle: n=26 mice, SS mouse model: n=29 mice. Mean ± SD. Unpaired two-tailed t-test. (D) H&E stained sections from the vehicle or DMXAA-treated animals. Treated animals showed minor lymphocyte infiltration and inflammation as focal peri-vascular/peri-ductal lymphocytic sialoadenitis adjacent to normal-looking acini. (E) The glandular damage was assessed by normalizing the weight of the submandibular gland (SMG) to the mouse’s body weight. Each dot represents the weight of one SMG. n=10 from 5 mice for both vehicle-treated and DMXAA-treated mice. (F–G) A comparison of total saliva secretion following 1 min stimulations at the indicated frequency (F) from the SMG of mice (Vehicle: n=8 mice, SS mouse model: n=6) and (G) from the parotid gland (PG) (Vehicle: n=7 mice, SS mouse model: n=5 mice). Mean ± SD. Two-way ANOVA with multiple comparisons. Source data is included in Figure 1—source data 1.
-
Figure 1—source data 1
Raw data of amounts of salivary secretion for individual animals.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig1-data1-v1.csv
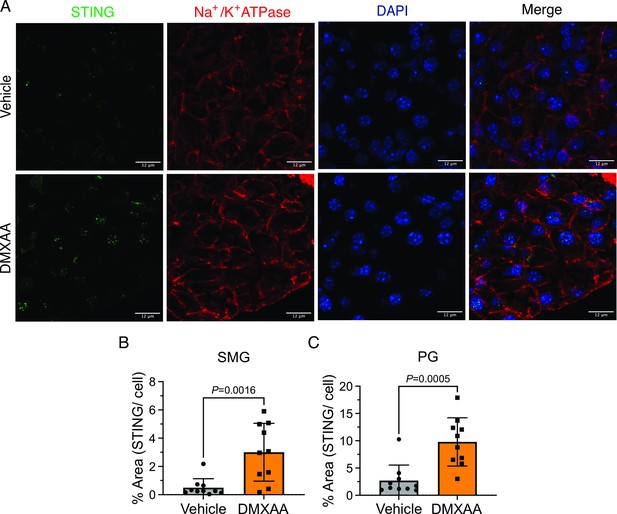
Up-regulation of stimulator of the interferon gene (STING) protein expression in both submandibular gland (SMG) and parotid gland (PG) treated with Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA).
Immunofluorescent staining in SMG tissue for STING (green), Na+/K+ ATPase (red), and DAPI for nucleus (blue). The upper panel is in vehicle-treated condition and the bottom panel is the SS mouse model. Scale bar: 12 μm. (B–C) STING protein expression was quantified by the percentage of a cell occupied by STING protein in (B) SMG and (C) PG. Vehicle, n=3 mice; SS mouse model: n=3 mice. Unpaired two-tailed t-test. Source data is included as Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Raw data for the amount of STING fluorescence per cell.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig1-figsupp1-data1-v1.csv
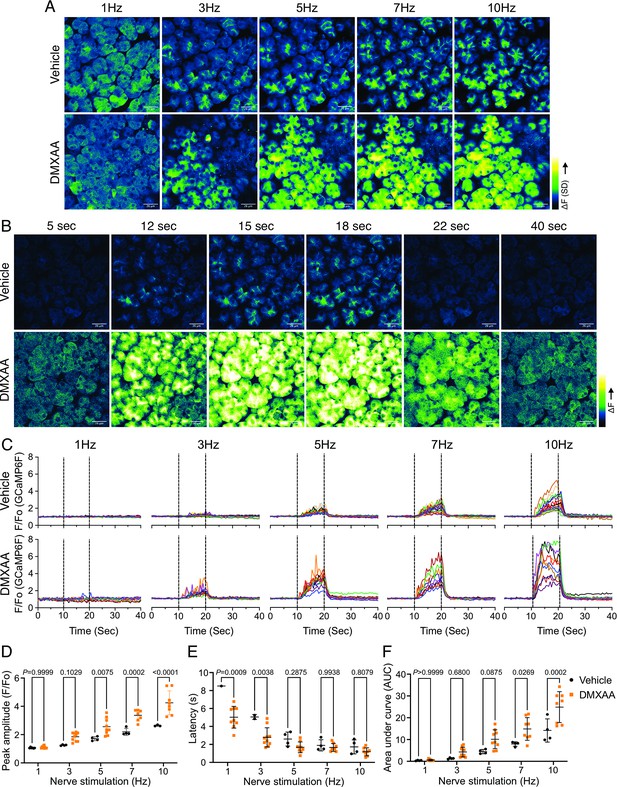
Augmented global Ca2+ signals in vivo in Sjögren's syndrome (SS) mouse model.
(A) Representative standard deviation images of Ca2+ signals during the 10 s of stimulation. Scale bar: 26 μm (B) The time-course of pseudo-color images of Ca2+ in response to 7 Hz stimulation. Scale bar: 26 μm (C) Representative cellular responses to stimulation at the indicated frequencies averaged from the entire cell. n=10 cells, one animal. (D) A comparison of peak Ca2+, (E) area under a curve, and (F) latency during each stimulation in submandibular gland (SMG). Each symbol represented the average response of ten cells from one view. Vehicle: n=3–6 from three mice; SS mouse model: n=8–10 from four mice. Mean ± SD. Two-way ANOVA with multiple comparisons. Source data is included in .
-
Figure 2—source data 1
Raw data for salivary secretion from individual animals.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig2-data1-v1.csv
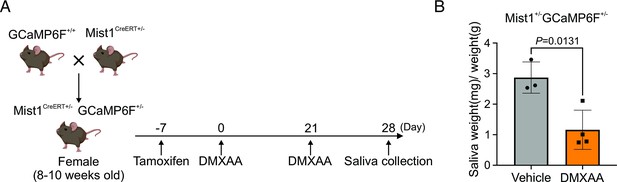
Deficiency in secretion in transgenic animals expressing GCamp6f following Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA) treatment.
(A) Schematic timeline for the generation of the Sjögren’s syndrome (SS) mouse model in the GCaMP6f expressing transgenetic mouse. Female mice received two subcutaneous doses of DMXAA on day 0 and day 21. The salivary gland function was assessed on day 28. (B) The gland function was evaluated by the weight of pilocarpine-induced saliva, normalized to each mouse’s weight. Vehicle = 3 mice, DMXAA = 4 mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included as Figure 2—figure supplement 1—source data 1.
-
Figure 2—figure supplement 1—source data 1
Raw data for the amount of saliva secretion for individual animals.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig2-figsupp1-data1-v1.csv
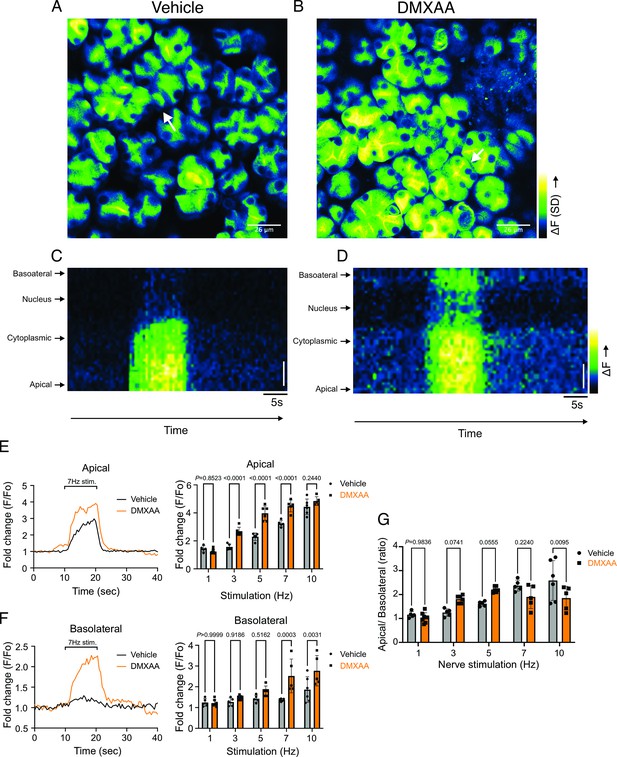
Disrupted spatial localization of Ca2+ signals in vivo in Sjögren's syndrome (SS) mouse model.
(A–B) A representative standard deviation image during the 7 Hz stimulation in (A) vehicle condition and (B) in the SS mouse model. Scale bar: 26 μm. An acinus is outlined by the white broken line and a line from apical to basal is shown in red in each standard deviation (SD) image. (C–D) A representative ‘kymograph’ image of consecutive lines stacked in space over time for 7 Hz stimulation in (C) vehicle condition and (D) SS mouse model. Time is encoded along the X-axis from left to right. Space is encoded along the Y-axis from the apical side (bottom) to the basolateral side (top). Scale bar: 3 μm. (E) Representative trace of Ca2+ signals at 7 Hz nerve stimulation in an apical ROI generated as the initial 2 mm of the scanned line over time (yellow line) in vehicle-treated (black) and DMXAA-treated (orange) mice. The changes in apical ROI fluorescence at the indicated frequencies were quantified as the maximal Ca2+ changes normalized to the basal intensity. (F) Representative trace of Ca2+ signals following 7 Hz nerve stimulation in a basolateral ROI generated as the final 2 mm of the scanned line (yellow line) over time in vehicle-treated (black) and DMXAA-treated (orange) mice. The changes in basolateral Ca2+ signals at the indicated frequencies were quantified by the maximal Ca2+ changes normalized to the basal intensity. (G) The ratio of the magnitude of Ca2+ signal on the apical vs. the basolateral ROI upon stimulation at the indicated frequencies. Vehicle: n=5–6 replicates from three mice; SS mouse model: n=5–8 replicates from four mice. Mean ± SD. Two-way ANOVA with multiple comparisons. Source data is included in Figure 3—source data 1.
-
Figure 3—source data 1
Raw data for individual animals.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig3-data1-v1.csv
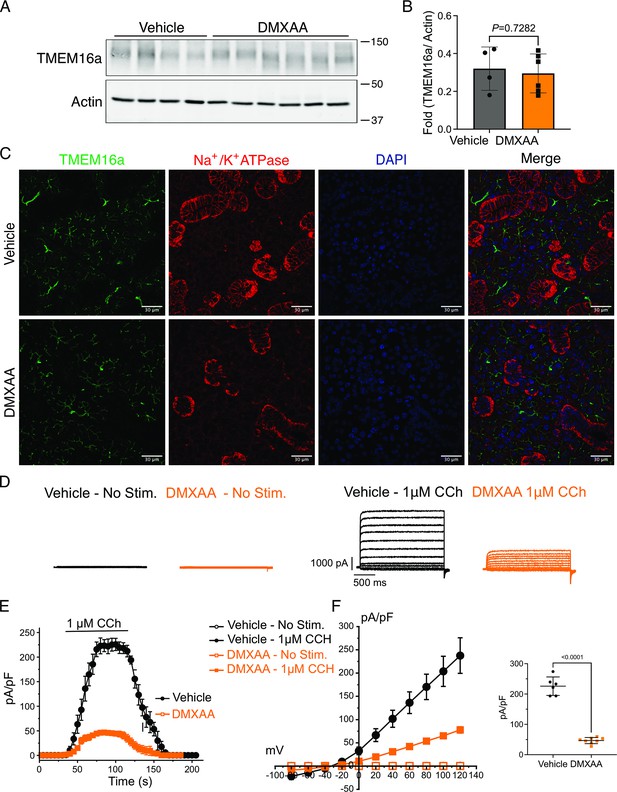
Attenuated whole-cell macroscopic Cl– currents induced by Carbachol (CCh) stimulation in Sjögren's syndrome (SS) mouse model.
(A) Western blotting showing the protein expression level of TMEM16a in the vehicle condition and the 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated SS mouse model. Actin is the internal control. (B) The quantification of TMEM16a protein expression normalized to the internal control, Actin. Vehicle, n=4 mice; SS mouse model: n=6 mice. (C) Immunofluorescent staining in submandibular gland (SMG) tissue for TMEM16a (green), Na+/K+ ATPase (red), and DAPI for nucleus (blue). The upper panel is from the vehicle-treated control and the bottom panel is from DMXAA-treated animals. Scale bar: 30 μm. Unpaired two-tailed t-test. (D) Cl- currents when cells were held at –50 mV and stepped from –80–120 mV in 20 mV increments. (E) Time-dependent Cl- current density changes in response to the Carbachol (CCh) in the isolated acinar cells in vehicle conditions and SS mouse model. (F) Current-voltage relationships were measured before and after the addition of CCh in vehicle conditions (n=three mice, 3–4 cells per mouse) and SS mouse model (n=three mice, 3–4 cells per mouse). TMEM16a currents in the treated mice were markedly reduced compared to the control mice. Black dots represent the vehicle-treated cells and orange squares represent DMXAA-treated cells. The open symbols represent no stimulation; the solid symbols represent CCh stimulation. Source data is included in Figure 4—source data 1 and 2.
-
Figure 4—source data 1
Raw densitometry and current magnitude data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig4-data1-v1.csv
-
Figure 4—source data 2
Original immunoblots.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig4-data2-v1.zip
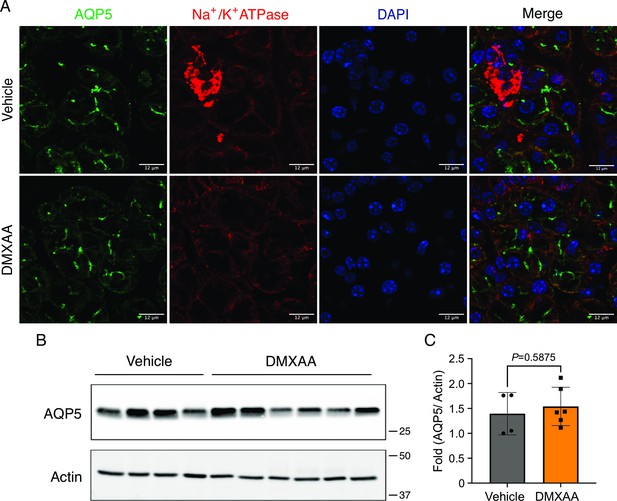
Aquaporin5 (AQP5), the water channel, remained the comparable expression and proper localization in submandibular gland (SMG) in the disease mouse model.
(A) Immunofluorescent staining in SMG tissue for AQP5 (green), Na+/K+ ATPase (red), and DAPI for nucleus (blue). The upper panel is from vehicle condition and the bottom panel is from the SS mouse model. Scale bar: 12 μm. (B) Western blotting showed the protein level of AQP5 in SMG from vehicle-treated control and disease mouse models. (C) The quantification of AQP5 normalized to internal control, Actin. Vehicle: n=4; SS mouse model: n=6. Mean ± SD. Unpaired two-tailed t-test. Source data is included as Figure 4—figure supplement 1—source data 1 and 2.
-
Figure 4—figure supplement 1—source data 1
Raw densitometry data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig4-figsupp1-data1-v1.csv
-
Figure 4—figure supplement 1—source data 2
Original immunoblots.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig4-figsupp1-data2-v1.zip
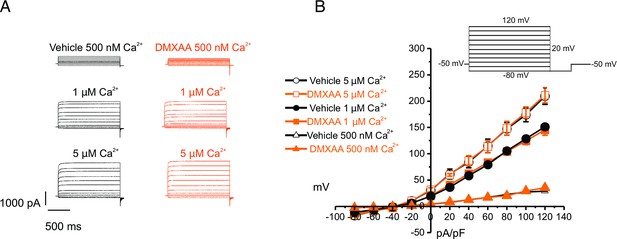
Increased [Ca2+]i is capable of restoring TMEM16a functionality to 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated mice.
(A) Cl- currents when cells were held at –50 mV and stepped from –80–120 mV in 20 mV increments. Either 0.5, 1, or 5 μM [Ca2+]i in the patch pipette elicited a similar magnitude of Cl- currents for both the treated (n=3 mice, 3–4 cells per mouse) and control mice (n=3 mice, 3–4 cells per mouse). (B) Current-voltage relationships for both populations were essentially identical. Vehicle and SS mouse model: n=3 mice, 3–4 cells per mouse.
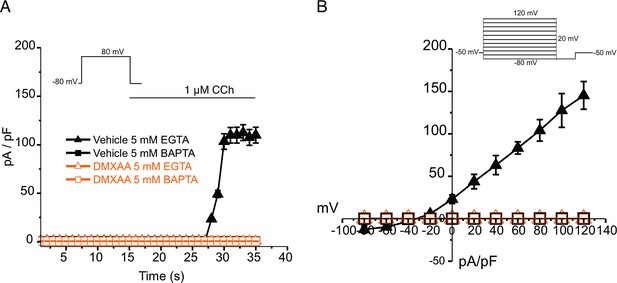
EGTA abolishes TMEM16a currents in 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated mice.
(A) Cl- currents in cells held at –80 mV and stepped to 80 mV with Carbachol (CCh) addition in EGTA (slow) and BAPTA (fast) buffered cells, respectively. (B) Current-voltage relationships were measured after the addition of CCh in 5 mM EGTA and 5 m M BAPTA-loaded isolated acinar cells from vehicle conditions (N=3 mice, 3–4 cells per mouse) and Sjögren’s syndrome (SS) mouse model (n=3 mice, 3–4 cells per mouse). No TMEM16a currents in acini in either vehicle or DMXAA-treated mice in cells buffered with BAPTA. Triangles represent the 5 mM EGTA condition; squares represent the 5 mM BAPTA condition. The solid black symbols represent the vehicle-treated cells and hollow orange symbols represent DMXAA-treated cells.
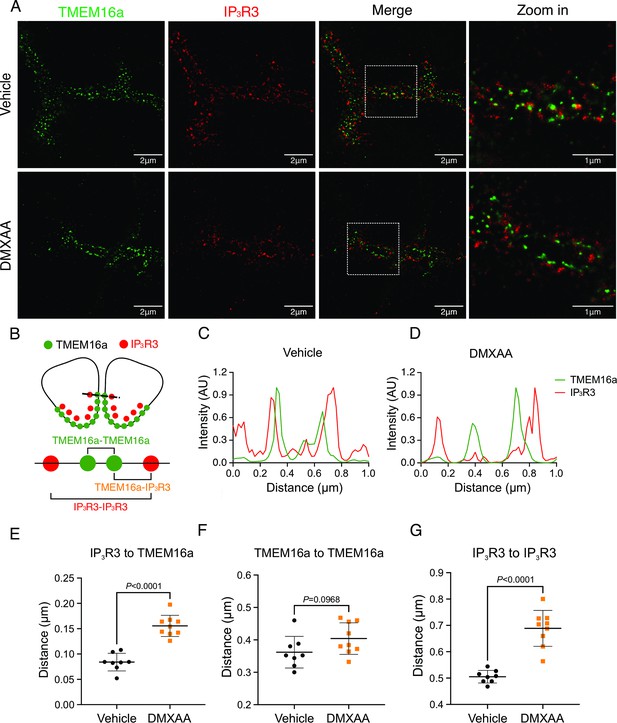
Disrupted proximity between TMEM16a and IP3R3 in the 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated Sjögren’s syndrome (SS) mouse model.
(A) Maximum projection of a STED z stack (1 μm) showing TMEM16a (green) and IP3R3 (red) in submandibular gland (SMG) tissue following Huygens deconvolution. The top panel represents the vehicle-treated control, and the bottom panel represents the SS mouse model. Scale bar: 2 μm. Zoomed images highlight the localization of TMEM16a and IP3R3 from the white square on the merged images. (B) Diagram illustrating the positioning of apical PM TMEM16a and apical IP3R3 in acinar cells. To analyze the proximity, a 1 μm reference line was drawn across the two parallel TMEM16a over two adjacent acinar cells with IP3R3 aligned vertically in the cytoplasm. (C–D) The representative traces of changes in fluorescence of TMEM16a (green) and IP3R3 (red) over the 1 μm distance. (E) Analysis of distance between TMEM16a and IP3R3 within cells. (F) Analysis of the distance between parallel TMEM16a on adjacent acinar cells. (G) Distance measurement of apical IP3R3 between two cells. Each symbol represents the mean of 5 examinations per image. Vehicle: n=8 replicates from 3 mice; SS mouse model: n=9 replicates from 3 mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included in Figure 7—source data 1.
-
Figure 7—source data 1
Raw distance measurements for individual line profiles.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig7-data1-v1.csv
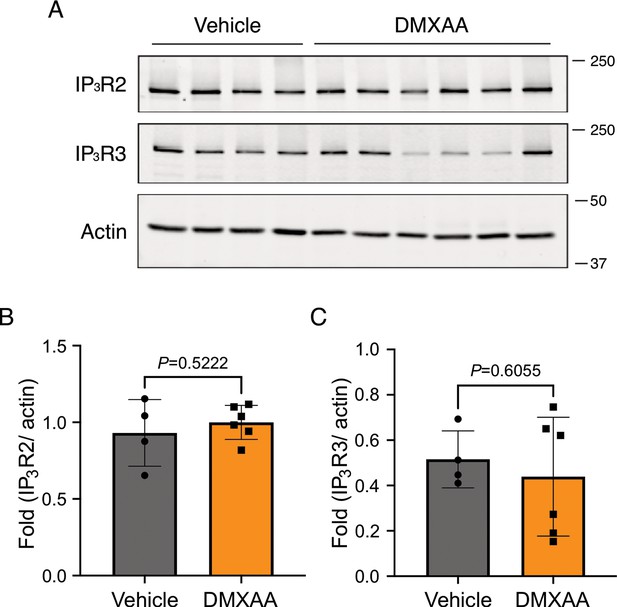
No significant alteration in IP3R protein levels in submandibular gland (SMG) in the 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated mouse model.
(A) Western blotting indicated the IP3R2 and IP3R3 protein levels from vehicle-treated control and Sjögren’s syndrome (SS) mouse models. Actin was probed as the internal control. (B) The quantification of IP3R2 and IP3R3 normalized to Actin, the internal control. Vehicle: n=4; SS mouse model: n=6. Mean ± SD. Unpaired two-tailed t-test. Source data is included as Figure 7—figure supplement 1—source data 1 and 2.
-
Figure 7—figure supplement 1—source data 1
Raw densitometry data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig7-figsupp1-data1-v1.csv
-
Figure 7—figure supplement 1—source data 2
Original immunoblots.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig7-figsupp1-data2-v1.zip
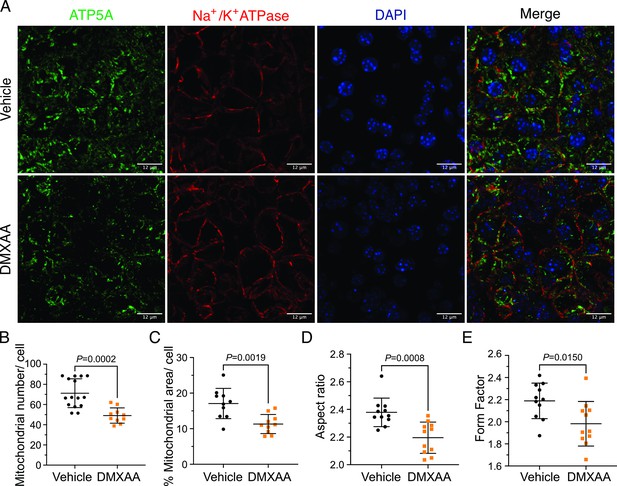
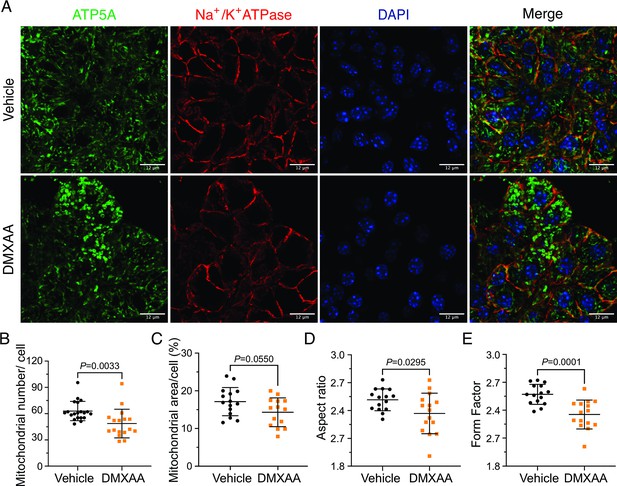
Mitochondrial alterations in acinar cells from the 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated Sjögren's syndrome (SS) mouse model.
(A) Immunofluorescent staining in submandibular gland (SMG) tissue for ATP5A (green), Na+/K+ ATPase (red), and DAPI for nucleus (blue). The upper panel is the vehicle, and the bottom panel is the SS mouse model. Scale bar: 12 μm. The mitochondrial content was quantified by (B) the mitochondrial number per acinar cell and (C) the percentage of area occupied by mitochondria per acinar cell. The mitochondrial morphology was analyzed by the (D) aspect ratio (AR) for the degree of mitochondrial tubular shape and (E) form factor (FF) for the degree of mitochondrial branching (complexity). In (B) to (E), black dots represent the vehicle condition, and orange squares indicate the SS mouse model. Each symbol represents the mean of 10 cells per image. Vehicle: n=10–15 from 3 mice; SS mouse model: n=10–11 from 3 mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included in Figure 8—source data 1.
-
Figure 8—source data 1
Raw mitochondrial morphology data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig8-data1-v1.csv
Mitochondrial alterations in the parotid gland of Sjögren's syndrome (SS) mouse model.
(A) Immunofluorescent staining in parotid gland (PG) tissue for ATP5A (green), Na+/K+ ATPase (red), and DAPI for nucleus (blue). The upper panel is from vehicle-treated animals and the bottom panel is from the SS mouse model. Scale bar: 12 μm. The mitochondrial content was quantified by (B) the mitochondrial number per acinar cell and (C) the percentage of area occupied by mitochondria per acinar cell. The mitochondrial morphology was analyzed by the (D) aspect ratio (AR) for the degree of mitochondrial tubular shape and (E) form factor (FF) for the degree of mitochondrial branching (complexity). In (B) to (E), black dots represent the vehicle condition, and orange squares indicate the SS mouse model. Each symbol represents the mean of 10 cells per image. Vehicle: n=20 and SS mouse model: n=15–17 from four mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included as Figure 8—figure supplement 1—source data 1.
-
Figure 8—figure supplement 1—source data 1
Raw mitochondrial morphology data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig8-figsupp1-data1-v1.csv
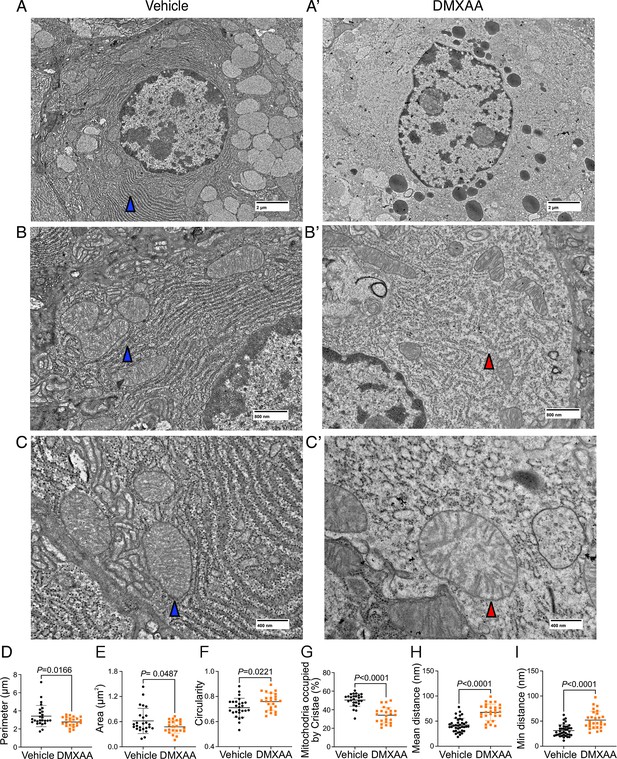
Ultrastructural analysis of mitochondria and endoplasmic reticulum (ER) in Sjögren's syndrome (SS) mouse model.
(A-C’') Images show mitochondrial cristae and ER structure by an electron microscopy (EM) at scales of (A-A’) 2 μm, (B-B’) 800 nm, and (C-C’) 400 nm. (D) Mitochondrial perimeter, (E) mitochondrial area, and (F) circularity were quantified by the shape description in ImageJ. (G) Quantification of mitochondrial cristae dispersion was evaluated by the percentage of cristae occupied in one mitochondrion. The (H) mean and (I) minimum proximity of ER and mitochondria were quantified by the plugin from http://sites.imagej.net/MitoCare/ in ImageJ. Vehicle: n=38 and SS mouse model: n=36 from 3 mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included in Figure 9—source data 1.
-
Figure 9—source data 1
Raw mitochondrial morphology data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig9-data1-v1.csv
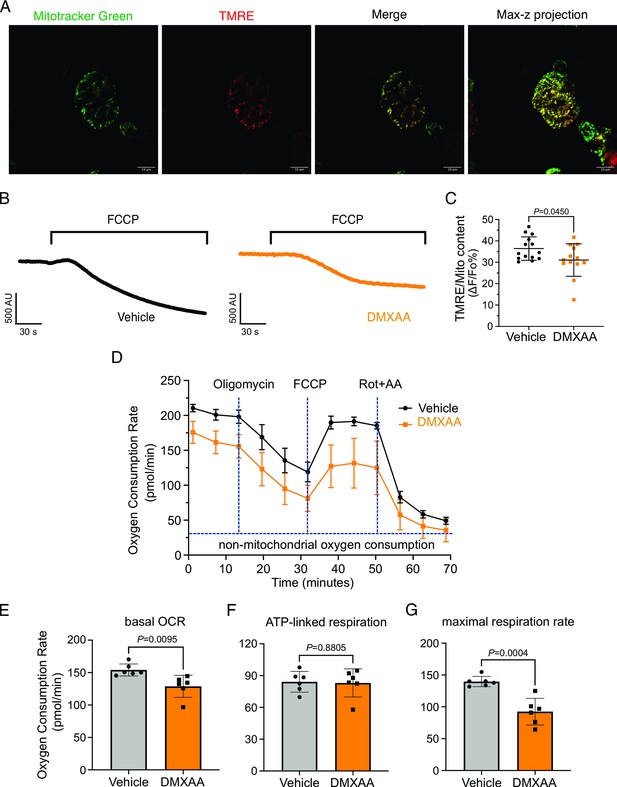
Mitochondrial bioenergetics are compromised in the 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated Sjögren's syndrome (SS) model.
(A) Mitochondria in the isolated acinar cells were labeled by the MitoTracker Green and co-stained with mitochondrial membrane potential dye, TMRE (red). The merged image shows the colocalization of both dyes, with maximal z-stack projection throughout the acinar cells. (B) Representative changes in mitochondrial membrane potential following FCCP-induced depolarization. The vehicle is shown in black; the SS mouse model is in orange. (C) The quantification was achieved by the difference of Tetramethylrhodamine, ethyl ester (TMRE) normalized to MitoTracker Green. Each dot is the mean of 10 cells from one experiment. Vehicle: n=14 and SS mouse model: n=13 from 3 mice. (D) Real-time mitochondrial respiration function was assessed in isolated acinar cells from the vehicle (black) and SS mouse model (orange) using the Seahorse XFe96 extracellular flux analyzer, in response to the pharmacological mito stress (oligomycin, FCCP, rotenone, and antimycin). Vehicle: n=59 and SS mouse model: n=32 from 6 mice. (E–G) Mitochondrial respiration function parameters were quantified by oxygen consumption rate (OCR) substracted the non-mitochondrial OCR for (E) basal respiration rate, (F) ATP-linked respiration rate, and (G) maximal respiration rate. Mean ± SD. Unpaired two-tailed t-test. Source data is included in Figure 10—source data 1.
-
Figure 10—source data 1
Raw mitochondrial bioenrgetics data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig10-data1-v1.csv
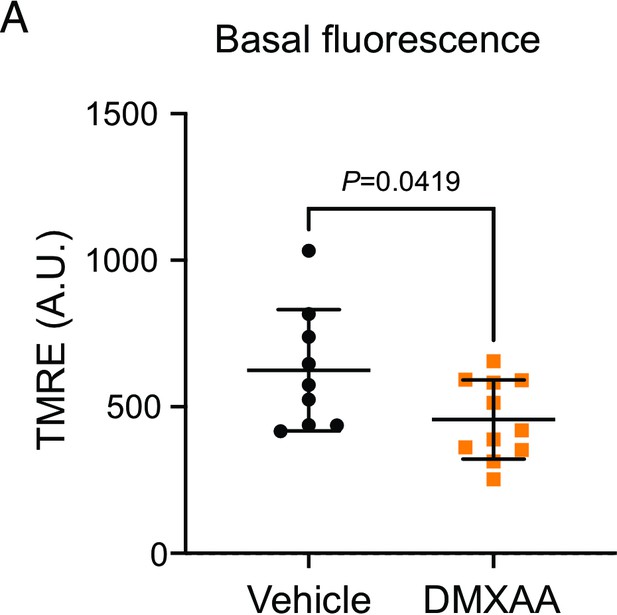
Reduction of the basal tetramethylrhodamine, ethyl ester (TMRE) fluorescence in 5,6-Dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA)-treated animals.
(A) The fluorescent intensity of TMRE loading in isolated acinar cells from vehicle-treated and DMXAA-treated mice. Black dots represent the vehicle condition, and orange squares indicate the SS mouse model. Each symbol represents the mean of 10 cells per image. Vehicle: n=9 and SS mouse model: n=11 from three mice. Mean ± SD. Unpaired two-tailed t-test. Source data is included as Figure 10—figure supplement 1—source data 1.
-
Figure 10—figure supplement 1—source data 1
Raw fluorescence data.
- https://cdn.elifesciences.org/articles/97069/elife-97069-fig10-figsupp1-data1-v1.csv
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (mouse) | C57BL/6 J | Jackson Laboratory | RRID:IMSR_JAX:000664 | 8–10 weeks-old female mice |
| Strain, strain background (mouse) | B6.129-Bhlha15tm3(cre/ERT2)Skz/J | Jackson Laboratory | RRID:IMSR_JAX:029228 | |
| Strain, strain background (mouse) | B6J.Cg-Gt(ROSA)26Sortm95.1(CAG-GCaMP6f)Hze/MwarJ | Jackson Laboratory | RRID:IMSR_JAX:028865 | |
| Chemical compound, drug | 5,6-dimethyl-9-oxo-9H-xanthene-4-acetic acid (DMXAA) | Vadimezan | GC16280 | Stock conc.: 10 mg/ml |
| Chemical compound, drug | Endotoxin 7.5% sodium bicarbonate solution | Sigma-Aldrich | S8761 | Working conc.: 5% |
| Chemical compound, drug | Pilocarpine | Millipore Sigma | P6503 | Stock conc.: 5.63 mg/ml Working conc: 1:100 |
| Antibody | Rabbit polyclonal TMEM16a antibody | Abcam | ab84115 | WB: 1:1000 IHC: 1:250 |
| Antibody | Mouse monoclonal alpha 1 Sodium Potassium ATPase antibody | Abcam | ab2872 | IHC: 1:250 |
| Antibody | Rabbit monoclonal alpha 1 Sodium Potassium ATPase antibody | Abcam | ab76020 | IHC: 1:500 |
| Antibody | Mouse monoclonal ATP5A antibody [15H4C4] | Abcam | ab14748 | IHC: 1:500 |
| Antibody | Rabbit monoclonal Aquaporin 5 antibody | Abcam | ab239904 | WB: 1:1000 IHC: 1:500 |
| Antibody | Rabbit monoclonal STING antibody (D2P2F) | Cell signaling Technology | #13647 | WB: 1:1000 IHC: 1:500 |
| Antibody | Rabbit polyclonal IP3R2 antibody (D2P2F) | PMID:33093175 | WB: 1:1000 IHC: 1:200 | |
| Antibody | Mouse monoclonal IP3R3 antibody (D2P2F) | BD Transduction Laboratory | 610313 | WB: 1:1000 IHC: 1:400 |
| Antibody | Mouse monoclonal Actin antibody | Millipore Sigma | A2228 | WB: 1:10000 |
| Antibody | Donkey anti-rabbit Alexa 488 | ThermoFisher Scientific | A-21206 | IHC: 1:500 |
| Antibody | Donkey anti-mouse Alexa 594 | ThermoFisher Scientific | A-21203 | IHC: 1:500 |
| Antibody | Goat anti-rabbit IgG (H&L) | Invitrogen | SA53557 | WB: 1:10000 |
| Antibody | Goat anti-mouse IgG (H&L) | Invitrogen | SA535521 | WB: 1:10000 |
| Chemical compound, drug | 4′,6-Diamidino-2-phenylindol (DAPI) | ThermoFisher Scientific | #62248 | IHC: 1:1000 |
| Chemical compound, drug | MitoTracker Green FM | InvitrogenTM | M7514 | Working conc.: 500 nM |
| Chemical compound, drug | Tetramethylrhodamine, ethyl ester (TMRE) | ThermoFisher Scientific | T669 | Working conc.: 20 nM |
| Chemical compound, drug | Oligomycin | Millipore Sigma | O4876 | Working conc.: 4 μg/ml |
| Chemical compound, drug | Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) | Millipore Sigma | C2920 | Working conc.: 2 μM |
| Chemical compound, drug | Rotenone | Millipore Sigma | R8875 | Working conc.: 2 μM |
| Chemical compound, drug | Antimycin | Millipore Sigma | A8674 | Working conc.: 2 μM |
| Other | Collagenase Type II | Worthington Biochemical | LS004204 | Working conc.: 0.2 mg/ml Reference: https://doi.org/10.1074/jbc.M406201200 |
| Other | Tamoxifen | Sigma-Aldrich | T5648 | Reference: https://doi.org/10.7554/eLife.66170v |
| Software, algorithm | FIJI/Image | Fiji (imagej.net) | ||
| Software, algorithm | Prism | GraphPad |





