Endogenous hydrogen peroxide positively regulates secretion of a gut-derived peptide in neuroendocrine potentiation of the oxidative stress response in Caenorhabditis elegans
Figures
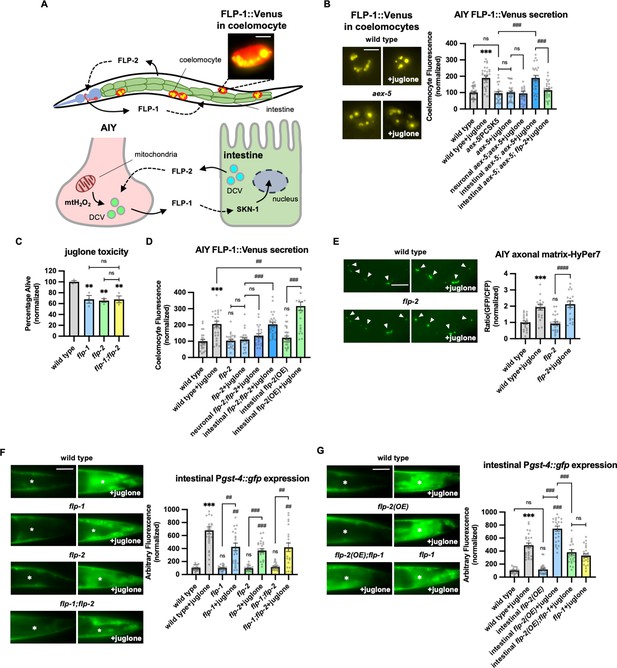
Peptidergic gut-to-neuron FLP-2 signaling potentiates the oxidative stress response.
(A) (Top) Schematic showing the positions of AIY, intestine, and coelomocytes of transgenic animals co-expressing FLP-1::Venus in the intestine and mCherry in coelomocytes. Representative image of the posterior coelomocyte that has taken up Venus into the endocytic compartment. Scale bar: 5 μM. (Bottom) Schematic showing FLP-1 and FLP-2 peptides as inter-tissue signals in gut-intestine regulation of the antioxidant response. (B) Representative images and quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Neuronal aex-5 denotes expression of aex-5 cDNA under the rab-3 promoter; intestinal aex-5 denotes expression of aex-5 cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=30, 30, 24, 30, 26, 30, 30 independent animals. Scale bar: 5 μM. (C) Average percentage of surviving young adult animals of the indicated genotypes after 16 hr recovery following 4 hr juglone treatment. Unlined ** denotes statistical significance compared to ‘wild type’. n=213, 156, 189, 195 independent biological samples over three independent experiments. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Neuronal flp-2 denotes expression of flp-2 gDNA under the rab-3 promoter; intestinal flp-2 denotes expression of flp-2 gDNA under the ges-1 promoter; intestinal flp-2(OE) denotes expression of flp-2 gDNA under the ges-1 promoter in wild-type animals. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=20, 20, 25, 20, 20, 20, 25, 22 independent animals. (E) Representative images and quantification of fluorescence of mitochondrial matrix-targeted HyPer7 in the axon of AIY following M9 or 300 μM juglone treatment for 10 min. Arrowheads denote puncta marked by mito::HyPer7 fusion proteins (excitation: 500 and 400 nm; emission: 520 nm). Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=24, 22, 25, 24 independent animals. Scale bar: 10 μM. (F) Representative images and quantification of average fluorescence in the posterior intestine of transgenic animals expressing Pgst-4::gfp after 1h M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region used for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined *** and ns denote statistical significance compared to ‘wild type’; unlined ## and ### denote statistical significance compared to ‘wild type+juglone’. n=25, 26, 25, 25, 25, 25, 25, 25 independent animals. Scale bar: 10 μM. (G) Representative images and quantification of average fluorescence in the posterior region of transgenic animals expressing Pgst-4::gfp after 1 hr M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘wild type+juglone’. n=23, 25, 25, 26, 24, 25 independent animals. Scale bar: 10 μM. (B–G) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 1—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig1-data1-v1.xlsx
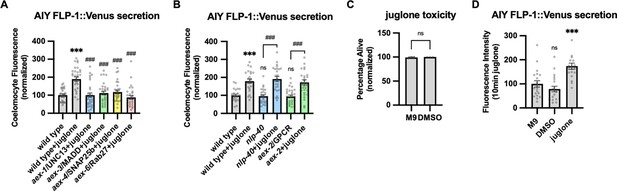
The effect of intestinal dense core vesicle (DCV) secretion mutations on FLP-1 release from AIY.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Unlined *** and ### denotes statistical significance compared to ‘wild type’. n=30, 30, 29, 30, 30, 30 independent animals. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Unlined *** and ns denote statistical analysis compared to ‘wild type’. n=24, 24, 25, 25, 30, 30 independent animals. (C) Average percentage of surviving young adult animals of the indicated genotypes after 16 hr recovery following 4 hr DMSO treatment. n=203, 174 independent biological samples over three independent experiments. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9, DMSO, or juglone treatment for 10 min. Unlined ns and *** denote statistical significance compared to ‘M9’. n=20, 20, 19 independent animals. (A–D) Data are mean values ± s.e.m. normalized to wild-type controls. (A, B, and D) ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test. (C) ns, not significant by unpaired t test with Welch’s correction.
-
Figure 1—figure supplement 1—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig1-figsupp1-data1-v1.xlsx
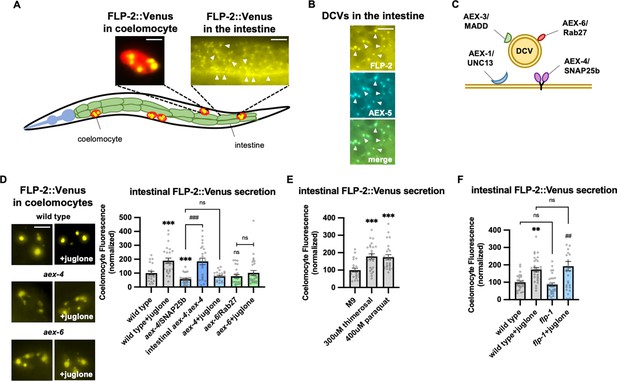
FLP-2 secretion from the intestine is stress regulated.
(A) Schematic showing the positions of intestine and coelomocytes of transgenic animals co-expressing FLP-2::Venus in the intestine and mCherry in coelomocytes. Representative images of the posterior coelomocyte that have taken up Venus into the endocytic compartment (scale bar: 5 μM) and the posterior intestinal region showing the distribution of FLP-2::Venus in puncta in the intestine are shown (scale bar: 15 μM). (B) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing FLP-2::Venus and AEX-5::mTur2 fusion proteins. Arrowheads denote puncta marked by both fusion proteins. Scale bar: 5 μM. (C) Schematic showing the locations of AEX-1/UNC13, AEX-3/MADD, AEX-4/SNAP25, and AEX-6/Rab27 relative to a dense core vesicle (DCV). (D) Representative images and quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone for 10 min. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=29, 25, 24, 30, 23, 30, 25, 25, 25 independent animals. Scale bar: 5 μM. (E) Quantification of average coelomocyte fluorescence of transgenic animals expressing FLP-2::Venus fusion proteins in the intestine following treatment with M9 buffer or the indicated stressors for 10 min. Unlined *** denotes statistical significane compared to ‘M9’. n=23, 25, 25 independent animals. (F) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Unlined ** denotes statistical significance compared to ‘wild type’; unlined ## denotes statistical significance compared to ‘flp-1’; a denotes statistical significance compared to ‘wild type+juglone’. n=30, 30, 30, 30 independent animals. (D–F) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 2—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig2-data1-v1.xlsx
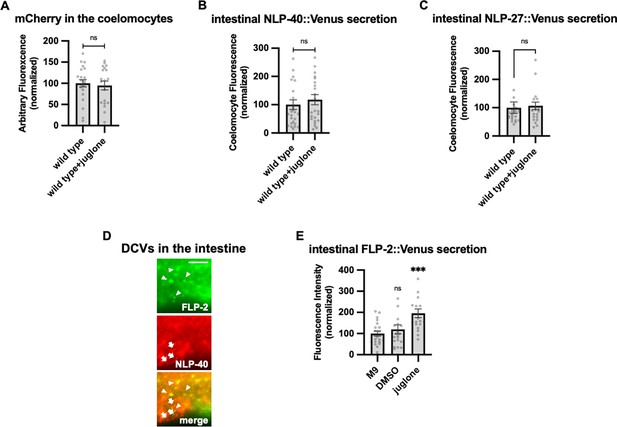
Specificity of juglone on intestinal peptide secretion, and FLP-2 and NLP-40 localization in the intestine.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants co-expressing FLP-2::Venus in the intestine (under the ges-1 promoter) and mCherry in the coelomocytes (under the ofm-1 promoter) following M9 or 300 μM juglone treatment for 10 min. n=23, 19 independent animals. (B) Quantification of average coelomocyte fluorescence of transgenic animals expressing NLP-40::Venus fusion proteins in the intestine following M9 or 300 μM juglone exposure for 10 min. n=25, 24 independent animals. (C) Quantification of average coelomocyte fluorescence of transgenic animals expressing NLP-27::Venus fusion proteins in the intestine following M9 or 300 μM juglone exposure for 10 min. n=23, 25 independent animals. (D) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing FLP-2::Venus fusion proteins (marked by arrowheads) and NLP-40::mTur2 fusion proteins (marked by arrows). Scale bar: 5 μM. (E) Quantification of average coelomocyte fluorescence of transgenic animals expressing FLP-2::Venus fusion proteins in the intestine following M9, DMSO, or 300 μM juglone exposure for 10 min. Unlined ns and *** denote statistical significance compared to ‘M9’. n=20, 20, 20 independent animals. (A–C and E) Data are mean values ± s.e.m. normalized to wild-type controls. (A–C) ns, not significant by unpaired t test with Welch’s correction. (E) ns, not significant by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 2—figure supplement 1—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig2-figsupp1-data1-v1.xlsx
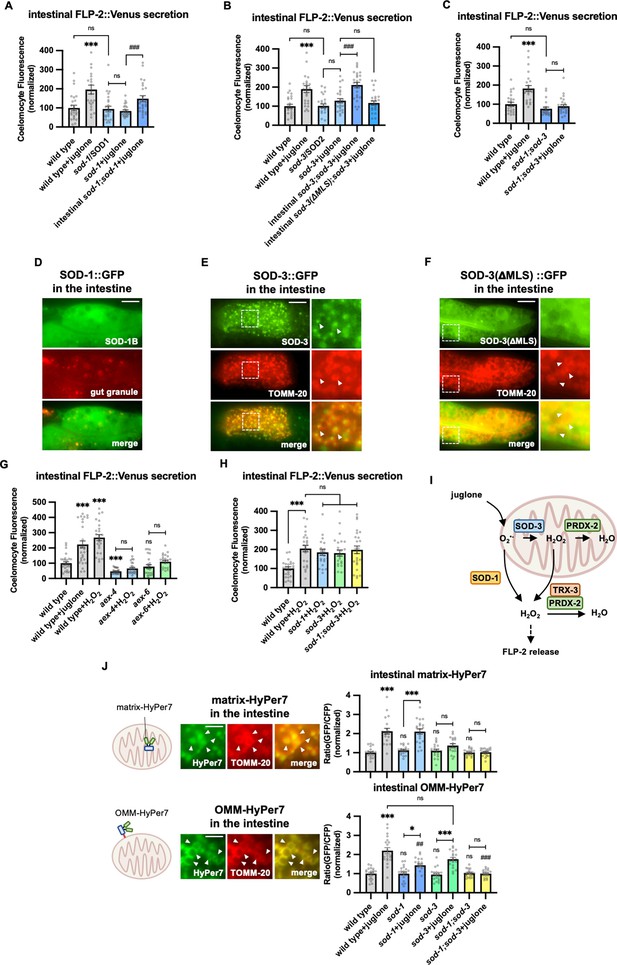
SOD-1/SOD-3 mediates endogenous H2O2 regulates FLP-2 release from the intestine.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal sod-1 denotes expression of sod-1b cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 22, 24, 24, 25 independent animals. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal sod-3 and sod-3(ΔMLS) denote intestinal expression of sod-3 cDNA and sod-3(ΔMLS) variants, which lacks the mitochondrial localization sequence, under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 25, 25, 25, 25, 25 independent animals. (C) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 25, 22, 25 independent animals. (D) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals expressing SOD-1b::GFP fusion proteins in contrast against autofluorescence of gut granules. Scale bar: 10 μM. (E) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing SOD-3::GFP and TOMM-20::mCherry (to target mitochondria) fusion proteins. Scale bar: 15 μM. (F) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing SOD-3(ΔMLS)::GFP and TOMM-20::mCherry fusion proteins. Scale bar: 15 μM. (G) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9, 300 μM juglone, or 1 mM H2O2 treatment for 10 min. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=29, 30, 25, 25, 25, 24, 25 independent animals. (H) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 1 mM H2O2 treatment for 10 min. n=independent animals. (I) Schematic showing that SOD-1 and SOD-3 mediate juglone-induced H2O2 production in promoting FLP-2 release, and the PRDX-2/TRX-3 system detoxifies excessive H2O2. (J) Schematic, representative images and quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing mitochondrial matrix targeted HyPer7 (matrix-HyPer7) or mitochondrial outer membrane targeted HyPer7 (OMM-HyPer7) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. Unlined ## and ### denote statistical significance compared to ‘wild type+juglone’. (Top) n=20, 20, 18, 20, 19, 19, 20, 20 independent animals. (Bottom) n=20, 20, 19, 20, 20, 20, 20, 20 independent animals. Scale bar: 5 μM. (A–C, G–H, and J) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, * p<0.05, ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 3—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig3-data1-v1.xlsx
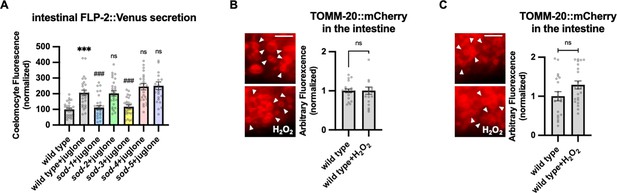
SODs function in juglone-induced FLP-2 release from the intestine and mitochondrial mCherry control.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or juglone treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### and ns denote statistical significance compared to ‘wild type+juglone’ n=29, 27, 29, 27, 25, 26, 24 independent animals. (B and C) Representative images and quantification of average fluorescence intensity of TOMM-20::mCherry proteins in transgenic animals co-expressing matrix-HyPer7 (B) or OMM-HyPer7 (C) following M9 or H2O2 treatment for 10 min. (B) Scale bar: 5 μM. n=20, 20 independent animals. (C) Scale bar: 5 μM. n=20, 22 independent animals. (A–C) Data are mean values ± s.e.m. normalized to wild-type controls. (A) ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test. (B and C) ns, not significant by unpaired t test with Welch’s correction.
-
Figure 3—figure supplement 1—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig3-figsupp1-data1-v1.xlsx
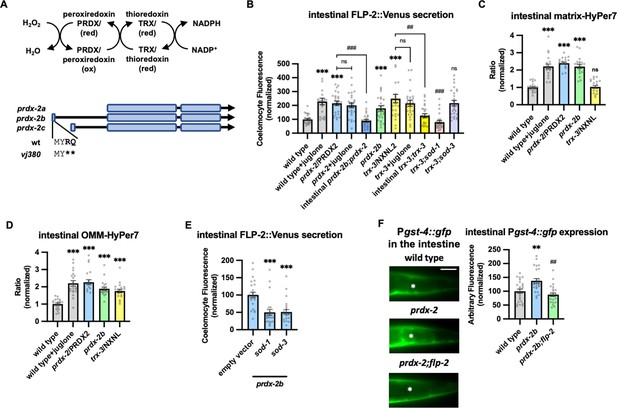
PRDX-2/PRDX and TRX-3/TRX regulate endogenous H2O2 and FLP-2 secretion.
(A) (Top) Schematic showing the PRDX/TRX system in H2O2 detoxification. (Bottom) Schematic showing the three isoforms of prdx-2 transcripts and vj380 allele of prdx-2b knockout. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal prdx-2b denotes expression of prdx-2b cDNA under the ges-1 promoter. Intestinal trx-3 denotes expression of trx-3 cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ## and ### denote statistical significance compared to ‘trx-3’. n=25, 23, 25, 25, 25, 25, 25, 25, 25, 25, 25 independent animals. (C and D) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (C) or OMM-HyPer7 (D) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. (C) n=20, 20, 20, 20, 20 independent animals. (D) n=20, 20, 20, 20, 20 independent animals. (E) Quantification of average coelomocyte FLP-2::Venus fluorescence of transgenic animals fed with RNA interference (RNAi) bacteria targeting the indicated genes following M9 treatment for 10 min. Unlined *** denotes statistical significance compared to ‘empty vector’. n=25, 23, 24 independent animals. (F) Representative images and quantification of average fluorescence in the posterior region of transgenic animals expressing Pgst-4::gfp after 1 hr M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined ** denotes statistical significance compared to ‘wild type’, unlined ## denotes statistical analysis compared to ‘prdx-2b’. n=25, 25, 25 independent animals. Scale bar: 10 μM. (B–F) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 4—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig4-data1-v1.xlsx
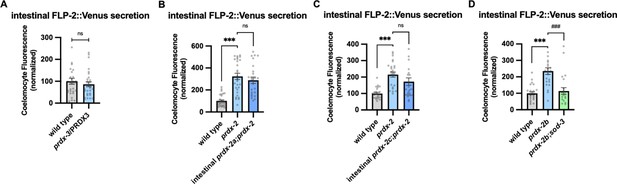
PRDX-2 intestinal rescue and mediates SOD-3-dependent regulation of FLP-2 release.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. n=30, 29 independent animals. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. Intestinal prdx-2a denotes expression of prdx-2a cDNA under the ges-1 promoter. n=30, 30, 25 independent animals. (C) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. Intestinal prdx-2c denotes expression of prdx-2c cDNA under the ges-1 promoter. n=25, 25, 25 independent animals. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. n=25, 23, 22 independent animals. (A–D) Data are mean values ± s.e.m. normalized to wild-type controls. (A) ns, not significant by unpaired t test with Welch’s correction. (B–D) ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 4—figure supplement 1—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig4-figsupp1-data1-v1.xlsx
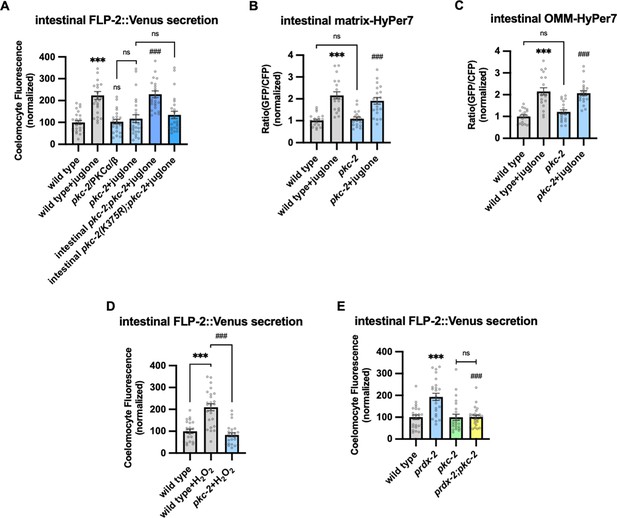
PKC-2/PKCα/β activation by H2O2 promotes FLP-2 secretion from the intestine.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal pkc-2 denotes expression of pkc-2b cDNA under the ges-1 promoter. Intestinal pkc-2b(K375R) denotes expression of pkc-2b(K375R) variants under the ges-1 promoter. Unlined *** and ns denote statistical significance compared to ‘wild type’; ### denotes statistical significance compared to ‘pkc-2+juglone’. n=24, 24, 25, 25, 25, 25 independent animals. (B and C) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (B) or OMM-HyPer7 (C) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical analysis compared to ‘pkc-2’. (B) n=20, 20, 19, 20 independent animals, (C) n=20, 20, 20, 20 independent animals. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 1 mM H2O2 treatment for 10 min. n=23, 25, 25 independent animals. (E) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘prdx-2’. n=25, 25, 25, 25 independent animals. (A–E) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 5—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig5-data1-v1.xlsx
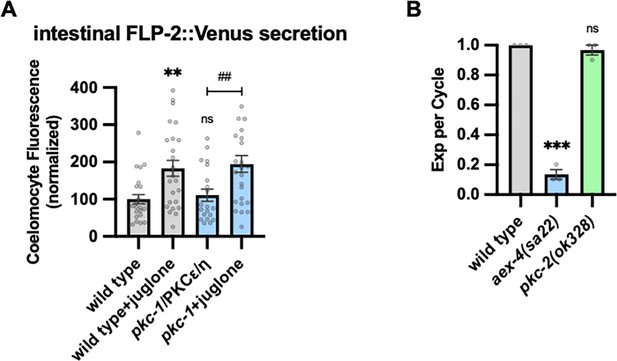
Juglone promotes FLP-2 release in pkc-1 mutants and expulsion analysis.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or juglone treatment for 10 min. Unlined ns and ** denote statistical significance compared to ‘wild type’. n=24, 25, 20, 25 independent animals. (B) Quantification of the number of expulsions (Exp) per defecation cycle in adult animals of the indicated genotypes. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=30, 30, 30, 30 in three independent animals. (A–B) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 5—figure supplement 1—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig5-figsupp1-data1-v1.xlsx
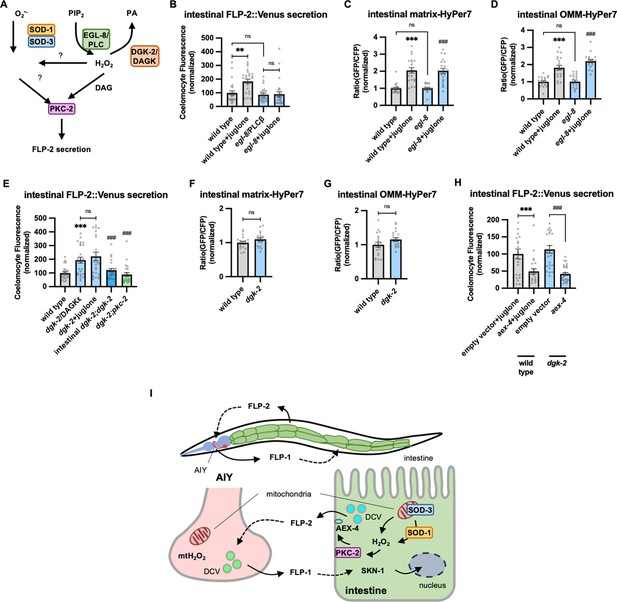
Diacylglycerol (DAG) promotes PKC-2-mediated FLP-2 secretion from the intestine.
(A) Schematic showing PLC and DGK mediates DAG metabolism and DAG functions in H2O2-mediated FLP-2 signaling. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or juglone treatment for 10 min. n=25, 25, 25, 25 independent animals. (C and D) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (C) or OMM-HyPer7 (D) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘egl-8’. (C) n=22, 20, 20, 21 independent animals, (D) n=20, 20, 20, 20 independent animals. (E) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal dgk-2 denotes expression of dgk-2a cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘dgk-2/DGKε’. n=25, 25, 25, 25, 24 independent animals. (F and G) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (F) or OMM-HyPer7 (G) with TOMM-20::mCherry following M9 treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. (F) n=20, 20 independent animals, (G) n=20, 20 independent animals. (H) Quantification of average coelomocyte fluorescence of the indicated transgenic animals fed with RNA interference (RNAi) bacteria targeting the indicated genes in the intestine following M9 treatment for 10 min. n=25, 24, 25, 30 independent animals. (I) (Top) Schematic showing the position of intestine and AIY neurons in FLP-1-FLP-2-mediated axis. (Bottom) Schematic showing endogenous H2O2 promotes PKC-2/AEX-4-mediated FLP-2 release from the intestine in FLP-1-FLP-2-regulated inter-tissue axis. (B–H) Data are mean values ± s.e.m. normalized to wild-type controls. (B–E and H) ns, not significant, ** p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test. (F and G) ns, not significant by unpaired t test with Welch’s correction.
-
Figure 6—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig6-data1-v1.xlsx
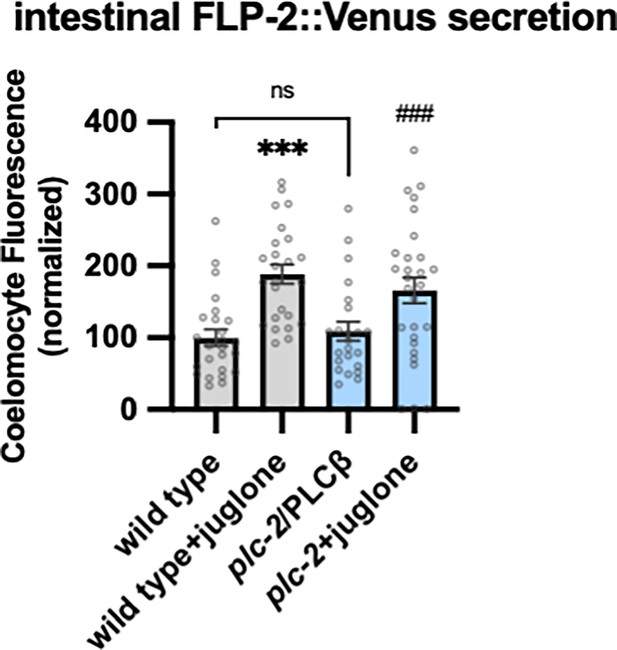
Juglone promotes FLP-2 release in plc-2 mutants.
Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or juglone treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘plc-2/PLCβ’. n=25, 25, 23, 28 independent animals. Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 6—figure supplement 1—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig6-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (C. elegans) | flp-1(ok2811) IV | CGC | OJ6555 | Mutant |
| Genetic reagent (C. elegans) | flp-2(ok3351) X | CGC | OJ5490 | Mutant |
| Genetic reagent (C. elegans) | flp-1(ok2811);flp-2(ok3351) | This paper | OJ10228 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22) X | CGC | OJ7466 | Mutant |
| Genetic reagent (C. elegans) | pkc-2(ok328) X | CGC | VC127 | Mutant |
| Genetic reagent (C. elegans) | vjIs150[pJQ60] | Jia and Sieburth, 2021 | OJ3614 | FLP-1::Venus |
| Genetic reagent (C. elegans) | aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5616 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1748[pJQ298]; aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5780 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1753[pJQ299]; aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5785 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1753[pJQ299];aex-5(sa23); flp-2(ok3351);vjIs150[pJQ60] | this paper | OJ6334 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351); vjIs150[pJQ60] | this paper | OJ5264 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2882[pJQ366];f lp-2(ok3351);vjIs150[pJQ60] | this paper | OJ8818 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302]; flp-2(ok3511);vjIs150[pJQ60] | this paper | OJ8813 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302];vjIs150 | this paper | OJ10229 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dvIs19[pAF15] | CGC | CL2166 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);dvIs19 | this paper | OJ2547 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351);dvIs19 | this paper | OJ10230 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);flp-2(ok3511);dvIs19 | this paper | OJ6544 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302];dvIs19 | this paper | OJ10231 | Obtained in from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302]; flp-1(ok2281);dvIs19 | this paper | OJ10232 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-1(sa9);vjIs150[pJQ60] | this paper | OJ5888 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-3(js815);vjIs150[pJQ60] | this paper | OJ5890 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22);vIs150[pJQ60] | this paper | OJ5891 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-6(sa24);vjIs150[pJQ60] | this paper | OJ5892 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | nlp-40(tm4085);vjIs150[pJQ60] | this paper | OJ5615 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-2(sa3);vjIs150[pJQ60] | this paper | OJ5889 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2035[pJQ305] | this paper | OJ6405 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3069[pDY10]; vjEx2035[pJQ305] | this paper | OJ9469 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22);vjEx2035[pJQ305] | this paper | OJ6409 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-6(sa24);vjEx2035[pJQ305] | this paper | OJ8345 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);vjEx2035[pJQ305] | this paper | OJ6641 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjIs40[pDS292] | this paper | OJ1002 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3263[pJQ370] | this paper | OJ10237 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3062[pDY14];vjEx2035[pJQ305] | this paper | OJ9567 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx2035[pJQ305] | this paper | OJ9797 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2814[pJQ419];sod-1 (tm783);vjEx2035[pJQ305] | this paper | OJ8588 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx0235[pJQ305] | this paper | OJ8341 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2910[pJQ389];sod-3 (tm760);vjEx2035[pJQ305] | this paper | OJ8933 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2973[pJQ408];sod-3 (tm760);vjEx2035[pJQ305] | this paper | OJ9106 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ10234 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3266[pJQ420] | this paper | OJ10243 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2993[pJQ407] | this paper | OJ9141 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2996[pJQ409(Pges-1::sod-3(∆MLS) cDNA::GFP)] | this paper | OJ9144 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3020[pJQ383] | this paper | OJ9230 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3014[pJQ411] | this paper | OJ9196 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx3020[pJQ383] | this paper | OJ9281 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx3020[pJQ383] | this paper | OJ9259 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx3020[pJQ383] | this paper | OJ10244 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx3014[pJQ411] | this paper | OJ9795 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx3014[pJQ411] | this paper | OJ9280 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx3014[pJQ411] | this paper | OJ10245 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-2(ok1030);vjEx2035[pJQ305] | this paper | OJ10238 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-4(gk101);vjEx2035[pJQ305] | this paper | OJ10239 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-5(tm1146);vjEx2035[pJQ305] | this paper | OJ10240 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169);vjEx2035[pJQ305] | this paper | OJ8991 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);vjEx2035[pJQ305] | this paper | OJ10251 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2926[pJQ381];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ8996 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);vjEx2035[pJQ305] | this paper | OJ9249 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3091[pJQ422];trx-3(tm2820); vjEx2035[pJQ305] | this paper | OJ9496 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);sod-1(tm783); vjEx2035[pJQ305] | this paper | OJ10252 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ10253 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169); vjEx3020[pJQ383] | this paper | OJ9237 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380); vjEx3020[pJQ383] | this paper | OJ10247 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820); vjEx3020[pJQ383] | this paper | OJ10249 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169); vjEx3014[pJQ411] | this paper | OJ10246 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380); vjEx3014[pJQ411] | this paper | OJ10248 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820); vjEx3014[pJQ411] | this paper | OJ10250 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);dvIs19 | this paper | OJ10254 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);flp-2(tm3351);dvIs19 | this paper | OJ10255 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-3(gk529);vjEx2035[pJQ305] | this paper | OJ10256 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3268[pJQ380];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ10258 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3270[pJQ399];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ10260 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ9250 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx2035[pJQ305] | this paper | OJ9682 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2828[pJQ376];pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ8682 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3131[pJQ446];pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ9657 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx3020[pJQ383] | this paper | OJ10279 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx3014[pJQ411] | this paper | OJ10280 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169);pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ8939 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-1(nj3);vjEx2035[pJQ305] | this paper | OJ10278 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx2035[pJQ305] | this paper | OJ9863 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx3020[pJQ383] | this paper | OJ10281 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx3014[pJQ411] | this paper | OJ10282 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx2035[pJQ305] | this paper | OJ10263 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx327[pJQ460];dgk-2(gk124); vjEx2035[pJQ305] | this paper | OJ10264 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ10266 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx3020[pJQ383] | this paper | OJ10283 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx3014[pJQ411] | this paper | OJ10284 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | plc-2(ok1761);vjEx2035[pJQ305] | this paper | OJ9809 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2936[pJQ382] | this paper | OJ9028 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351);vjEx2936[pJQ382] | this paper | OJ10595 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Prab-3::aex-5 cDNA (plasmid) | this paper | pJQ298 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::aex-5 cDNA | this paper | pJQ299 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Prab-3::flp-2 gDNA | this paper | pJQ366 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::flp-2 gDNA | this paper | pJQ302 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::aex-5::mTur2 | this paper | pDY10 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::flp-2::Venus | this paper | pJQ305 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::nlp-27 gDNA::Venus | this paper | pJQ370 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::nlp-40::mTur2 | this paper | pDY14 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-1a cDNA | this paper | pJQ419 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3 cDNA | this paper | pJQ389 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3(∆MLS) cDNA | this paper | pJQ408 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-1a cDNA::GFP | this paper | pJQ420 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3 cDNA::GFP | this paper | pJQ407 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3(∆MLS) cDNA::GFP | this paper | pJQ409 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::MLS::HyPer7 | this paper | pJQ383 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::tomm-20::HyPer7 | this paper | pJQ411 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2b cDNA | this paper | pJQ381 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::trx-3 cDNA | this paper | pJQ422 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2a cDNA | this paper | pJQ380 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2c cDNA | this paper | pJQ399 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::pkc-2b cDNA | this paper | pJQ376 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::pkc-2(K375R) cDNA | this paper | pJQ446 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::dgk-2a cDNA | this paper | pJQ460 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pttx-3::MLS::HyPer7 | this paper | pJQ382 | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGCTAGCAAAAATGAAATTAATTTTCCTGCTTTTGCTTTTTGG | this paper | aex-5_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGGTACCTTATGACATTGTTCCCACCACT | this paper | aex-5_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGCAAGTTTCTGGAATCCTATCTGC | this paper | flp-2_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATTGGA AGTCGTAATCTGGCAGC | this paper | flp-2_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGGTACCTTATGACATTGTTCCCACCACT | this paper | aex-5_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCACCGGTTTGGAAGTCGTAATCTGGCAGCGG | this paper | flp-2_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGATTTCCACTTCTTCACTTCTTATCCTT | this paper | nlp-27_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCACCGGTCTTTCCCCATCCACCGTATCC | this paper | nlp-27_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTT TATGAATCTTCTCACTCAGGTCTCC | this paper | sod-1a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTCACTGGGGAGCAGCGAGAG | this paper | sod-1a_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGCTGCAATCTACTGCTCGC | this paper | sod-3_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATTGTCGAGCATTGGCAAATCT | this paper | sod-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGAAGCACACTCTCCCAGA | this paper | sod-3(∆MLS)_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCCGGGCTGGGGAGCAGCGAGAGCAA | this paper | sod-1_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCCGGGTTGTCGAGCATTGGCAAATCTC | this paper | sod-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTA TAGACAGATGTCGAAAGCATTC | this paper | prdx-2b_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTAGTGCT TCTTGAAGTACTCTTGG | this paper | prdx-2a/b/c_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGG CTAAGAACTTTTTCTCCGGA | this paper | trx-3_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATGCACGGATTCTCTCGAGATT | this paper | trx-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTCGAAAGCATTCATCGGAA | this paper | prdx-2a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATG TCTCTCGCTCCAAAGATG | this paper | prdx-2c_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTCGTTGAGCACGAACAGC | this paper | pkc-2b_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGATATCTCACGGTTCTACATCTTTGACATAAAAC | this paper | pkc-2b_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | ATTTCCTCACTGTTCTTGGAAGAGGATCGTTTG | this paper | pkc-2b(K375R)_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | ACACTTTTCCAAACGATCCTCTTCCAAGAACA | this paper | pkc-2b(K375R)_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGGAAAT GGACGTGTATGATGAATTATTG | this paper | dgk-2a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTAGAAG AACATCCCACATCCGG | this paper | dgk-2a_R | Obtained from Derek Sieburth lab |
| Software, algorithm | Etho | James Thomas Lab | Defecation motor program analysis | http://depts.washington.edu/jtlab/software/otherSoftware.html |
| Software, algorithm | Metamorph 7.0 | Universal 709 Imaging/Molecular Devices | Image capture | |
| Software, algorithm | GraphPad Prism 9 | Prism | Statistical analysis |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/97503/elife-97503-mdarchecklist1-v1.pdf
-
Supplementary file 1
Strains, transgenic lines, and plasmids used in this study.
- https://cdn.elifesciences.org/articles/97503/elife-97503-supp1-v1.docx





