Pathogenic Huntingtin aggregates alter actin organization and cellular stiffness resulting in stalled clathrin-mediated endocytosis
Figures
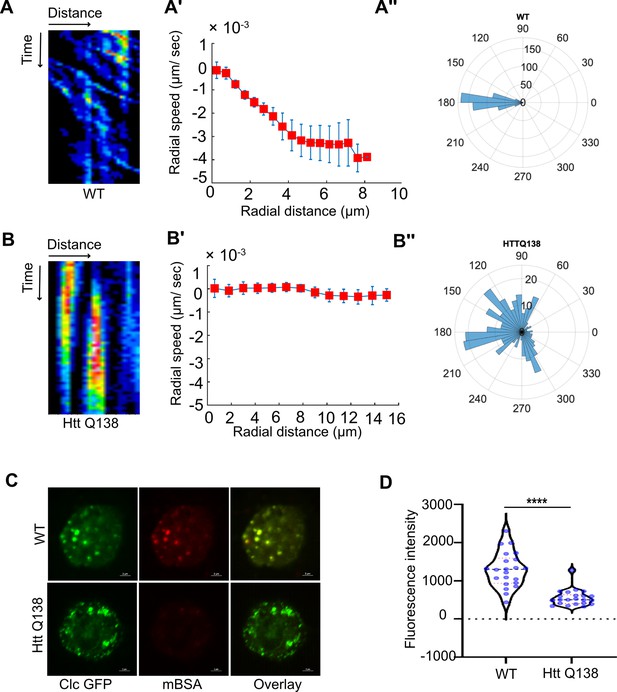
Clathrin mediated endocytosis and clathrin coated structure dynamics are compromised in the presence of pathogenic Huntingtin polyQ aggregates.
(A) Kymograph showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in WT hemocytes. X-axis represents distance and Y-axis represents time. (A’) Radial speed (µm/ sec) of the CCSs in WT hemocytes as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis (see Materials and methods for details). (A’’) Polar histogram of distribution of the flow-field directions obtained from PIV analysis relative to the polar direction (see Materials and methods for details). The angles are sharply distributed around a value of 180°, showing the centripetal movement of CCSs. (B) Kymograph showing stalled movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in HTT Q138 hemocytes. (B’) Graph showing radial speed (µm/ sec) of the CCSs in HTT Q138 hemocytes. (B’’) Polar histogram of flow-field directions obtained similar to the WT case in A’’ gives a broad distribution of the angles, indicating the absence of any directional centripetal movement of CCSs in presence of HTT Q138. (C) Internalization of mBSA in WT and HTT Q138 cells. Clathrin puncta are shown in green and mBSA in the red channel. Scale bar 2 µm. (D) Graph shows the quantification of internalized mBSA. Y-axis represents the intensity of mBSA internalized under different conditions. N=20 cells. p-value <0.0001 (Mann-Whitney test).
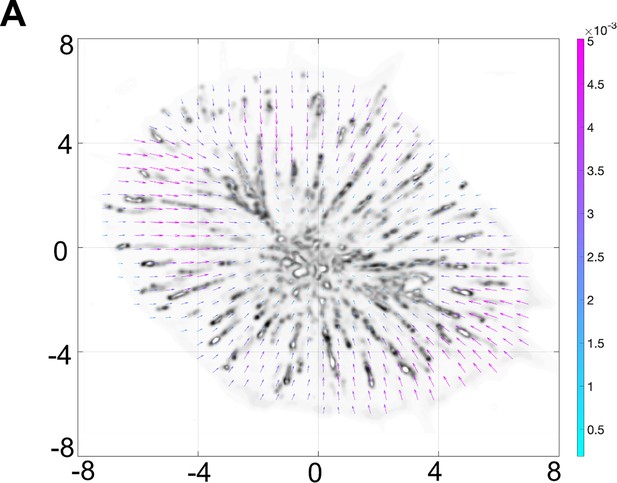
Time-averaged CCS flow-field obtained from the PIV analysis for wild type cells.
(A) Time-averaged CCS flow-field obtained from the PIV analysis for wild type cells. The flow-field vectors are plotted on top of the corrected clathrin intensity shown in gray-scale. The color of the vectors represents the vector magnitudes (see color-bar for the exact values in um/sec).
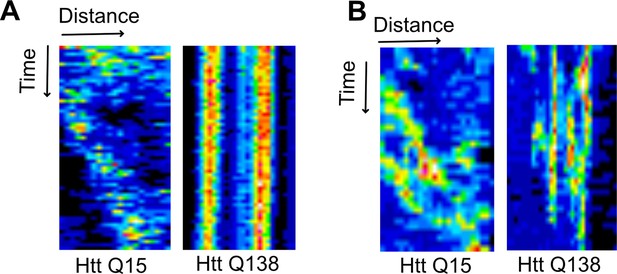
CCS movement is stalled in the presence of pathogenic HTT aggregates in mammalian cells.
Kymograph showing movement of CCSs in presence of HTT Q15 while movement is stalled in the presence of HTT Q138 in (A) HEK 293T and (B) SH-SY5Y cells, respectively.
Video showing time lapse imaging of a HTT Q15 hemocyte expressing clathrin light chain tagged with GFP over 5 mins at 5 s intervals.
Video showing time lapse imaging of an HTT Q138 hemocyte expressing clathrin light chain tagged with GFP over 5 min at 5 s intervals.
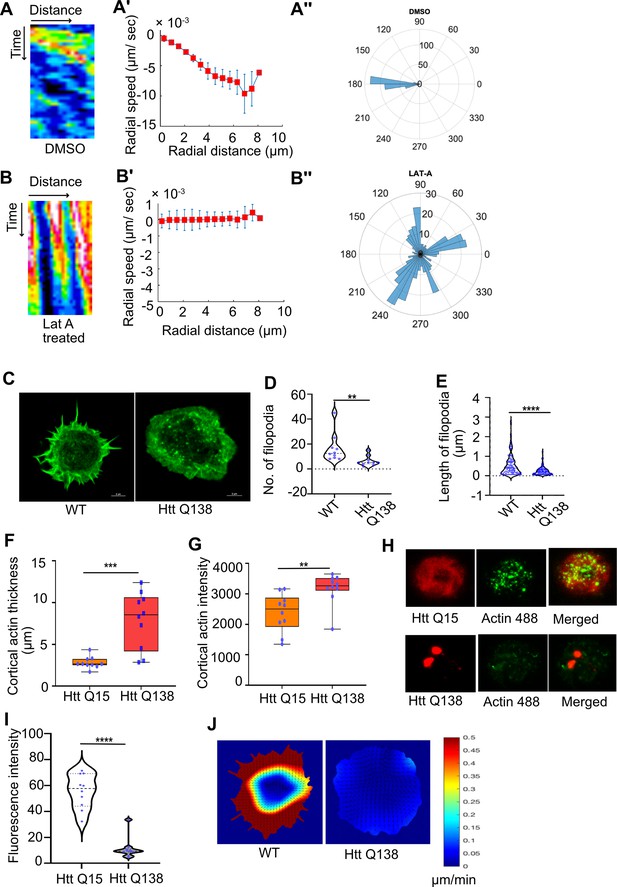
Presence of Huntingtin polyQ aggregates negatively affect Actin dynamics.
(A) Kymographs showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in DMSO treated cells. (A’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis. (A’’) Polar histogram of distribution of the flow-field directions obtained from PIV analysis relative to the polar direction (see Materials and methods for details). The angles are sharply distributed around a value of 180°, showing the centripetal movement of CCSs. (B) Kymographs showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in LatA-treated cells. (B’) Radial speed (µm/ sec) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis shows stalled movement of CCSs (B’’). Polar histogram of flow-field directions gives a broad distribution of angles, indicating the absence of any directional centripetal movement of CCSs upon LatA treatment. (C) Representative micrograph showing filopodia formation in HTT Q15, whereas no filopodia are seen in HTT Q138 expressing cells. Scale bar 5 µm. (D) Graphs showing the quantification of the number of filopodia in WT and HTT Q138 expressing cells. Number of cells = 10, p-value = 0.001 and (E) shows quantification of length of filopodia in WT and HTT Q138 expressing cells. Number of cells = 10, p-value <0.0001 (Mann-Whitney test). (F, G) Box plot showing increase in cortical thickness and actin intensity in cortical region of cells respectively. Number of cells = 10. p-value = 0.0015, p-value = 0.0003 (Mann-Whitney test). (H) Micrograph showing barbed-end labelling of actin in hemocytes expressing either HTT Q15 or HTT Q138. HTT Q15/Q138 are shown in red, whereas barbed ends labeled with actin are shown in the green channel. (I) Violin plot shows quantification of labeled barbed end in HTT Q15 and HTT Q138 expressing cells. Number of cells = 10. p-value <0.0001 (Mann-Whitney test). (J) Representative images showing actin flow in wild type and HTT Q138 expressing cells, obtained from PIV analysis.
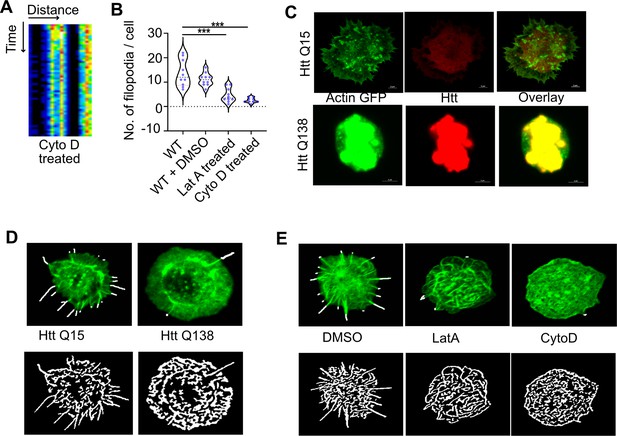
Presence of Huntingtin polyQ aggregates alters Actin dynamics.
(A) Kymograph showing stalled movement of CCSs upon treatment with CytoD. (B) Graph showing quantification of number of filopodia in WT, DMSO (vehicle control), LatA and CytoD-treated cells. Number of cells = 10. p-value = 0.0025, p-value <0.0001 (Kruskal- Wallis test followed by post hoc Dunn’s multiple comparisons test). (C) Micrograph showing sequestration of actin-GFP in HTT Q138 aggregates in Drosophila S2 cells. HTT Q15 or HTT Q138 are tagged with mRFP. HTT Q15 does not sequester actin and filopodia like projections are seen in HTT Q15 expressing cells. Scale bar 2 µm. (D) Micrograph showing representative images obtained from Filoquant for quantification of number and length of filopodia in Htt Q15 and Htt Q138 conditions. (E) Micrograph showing representative images obtained from Filoquant for quantification of number and length of filopodia in DMSO, LatA, and CytoD-treated WT hemocytes.
Video showing time lapse imaging of a WT hemocyte expressing Lifeact tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte expressing Lifeact tagged with GFP in presence of HTT Q138 over 5 min at 5 s intervals.
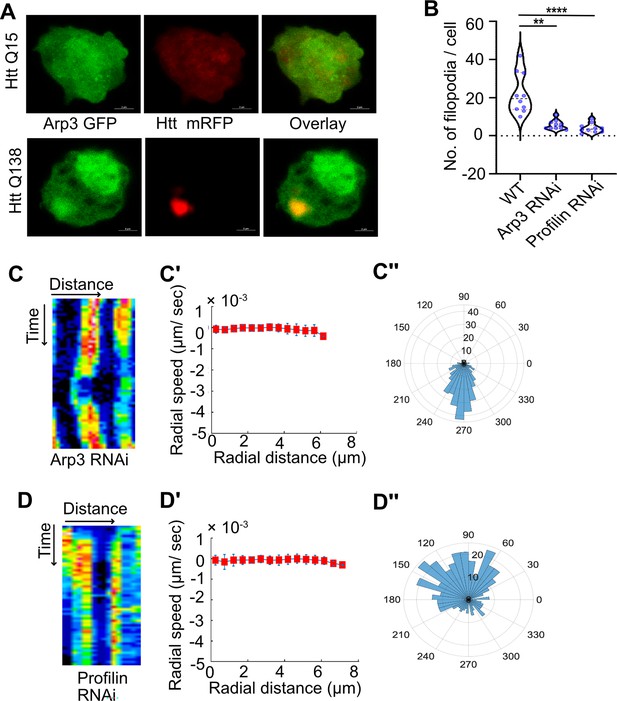
Knockdown of components of the Arp2/3 complex or Profilin result in disruption of CCS movement.
(A) Representative micrographs showing sequestration of Arp3 in HTT Q138 aggregates. Arp3 is shown in green and HTT Q15 /HTT Q138 - RFP is shown in red channel. (B) Graph showing quantification of number of filopodia upon Arp3 or Profilin knockdown. Number of cells = 10. p-value = 0.0025, p-value <0.0001 (Kruskal-Wallis test followed by post hoc Dunn’s multiple comparisons test). (C) Kymograph showing stalled movement of CCSs by live cell imaging of clathrin light chain tagged with GFP upon Arp3 knockdown. (C’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis shows compromised movement of CCSs upon Arp3 knockdown. (C’’) Polar histogram of flow-field directions gives a broad distribution of the angles, indicating the absence of any directional centripetal movement of CCSs upon Arp3 knockdown. (D) Kymographs showing stalled movement of CCSs by live cell imaging of clathrin light chain tagged with GFP upon Profilin knockdown. (D’, D’’) PIV analysis shows stalled CCS movement and loss in directionality under Profilin knockdown conditions.
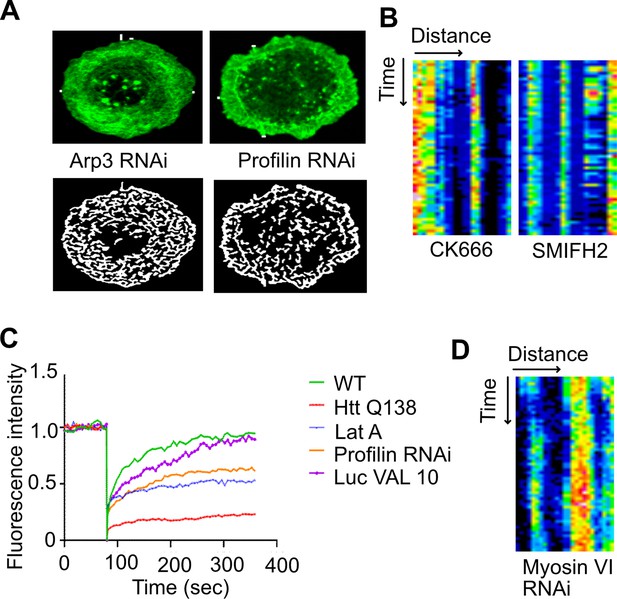
CCS movement is disrupted on blocking actin redmodeling.
(A) Micrograph showing representative images obtained from Filoquant for quantification of number and length of filopodia upon Arp3 or Profilin knockdown. (B) Kymograph showing stalled movement of CCSs upon treatment with CK666 (Arp2/3 inhibitor) or SMIFH2 (Formin inhibitor). (C) Fluorescence intensity of Clc-GFP upon FRAP of individual CCSs. Y-axis depicts GFP intensity and X-axis represents time in seconds. Recovery of CLC GFP is seen at individual CCSs in WT and Luc VAL 10 (control for profilin KD). However, HTT Q138 expressing cells, Lat-A-treated cells or profilin knockdown cells only show partial recovery. (D) Kymograph showing stalled movement of CCSs upon knocking down Myosin VI.
Video showing time lapse imaging of a WT hemocyte expressing Tubulin tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing HTT Q138 aggregates expressing Tubulin tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals upon myosin VI knockdown condition.
Video showing time lapse imaging of a hemocyte expressing Lifeact tagged with GFP over 5 min at 5 s intervals upon myosin VI knockdown condition.
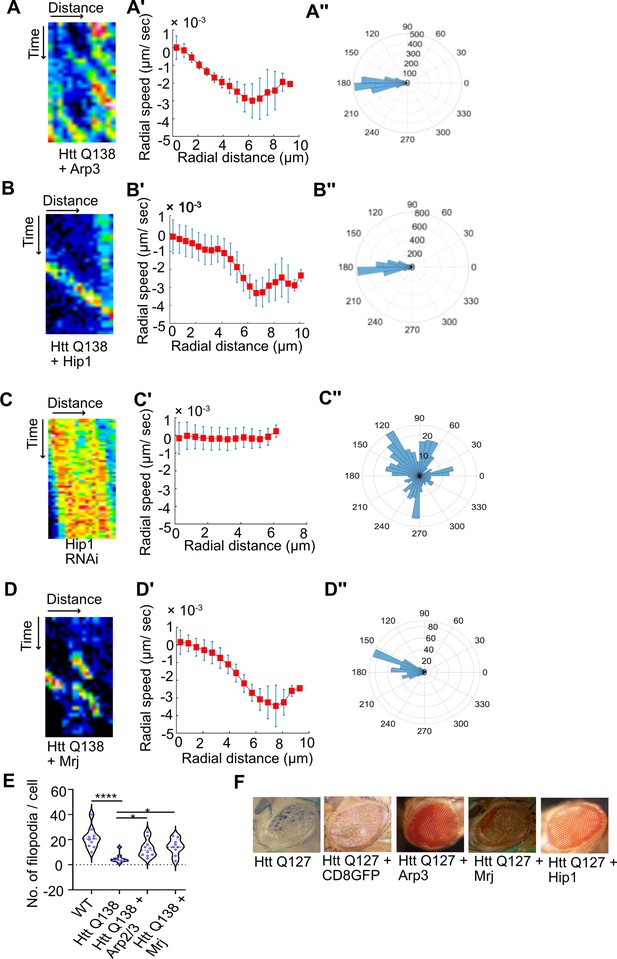
Overexpression of components of the Arp2/3 complex or Hip1 rescue CCS movement and neurodegeneration even in the presence of pathogenic HTT.
(A) Kymograph showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in HTT Q138 +Arp3 condition. (A’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis. (A’’) Polar histogram of distribution of the flow-field directions obtained from PIV analysis relative to the polar direction are shown. The angles are sharply distributed around a value of 180°, showing the centripetal movement of CCSs. (B) Kymograph showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in HTT Q138 +Hip1 condition. (B’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis. (B’’) Polar histogram of distribution of the flow-field directions obtained from PIV analysis relative to the polar direction. The angles are sharply distributed around a value of 180°, showing the centripetal movement of CCSs in HTT Q138 +Hip1 condition. (C) Kymograph showing stalled movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in Hip1 knockdown cells. (C’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis shows compromised movement of CCSs in Hip1 knockdown cells. (C’’) Polar histogram of flow-field directions gives a broad distribution of the angles, indicating the absence of any directional centripetal movement of CCSs upon Hip1 knockdown. (D) Kymograph showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP in HTT Q138 +Mrj cells. (D’) Radial speed (µm/ s) of the CCSs as a function of radial distance (in µm) from the cell center obtained from time-averaged PIV analysis in HTT Q138 +Mrj cells. (D’’) Polar histogram of distribution of the flow-field directions obtained from PIV analysis relative to the polar direction shows the angles are sharply distributed around a value of 180°, showing the centripetal movement of CCSs. (E) Violin plot showing quantification of number of filopodia in WT, HTT Q138, HTT Q138 +Arp3 and HTT Q138 +Mrj cells. Number of cells = 10, p-value = 0.0309 for HTT Q138 +Mrj and p-value = 0.0154 for HTT Q138 +Arp3 compared to HTT Q138, p-value <0.0001 (Kruskal-Wallis test followed by post hoc Dunn’s multiple comparisons test). (G) Representative micrograph showing Drosophila eye expressing HTT Q127, HTT Q127 +CD8 GFP, HTT Q127 +Arp3, HTT Q127 +Mrj and HTT Q127 +Hip1.
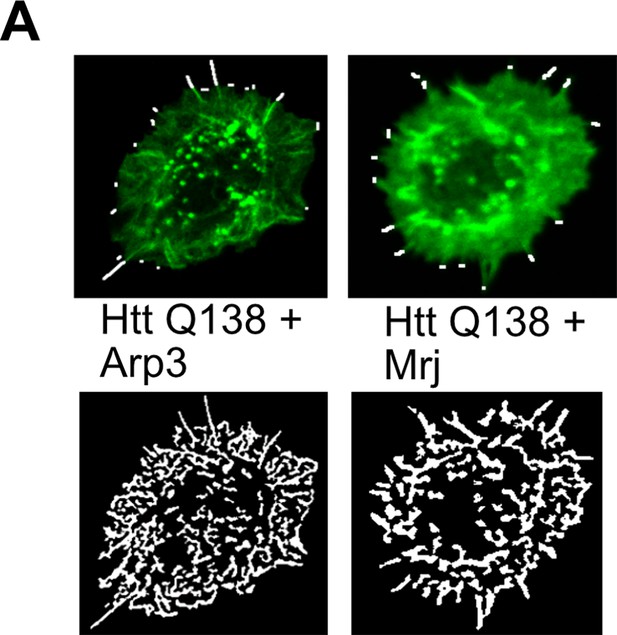
Micrograph showing representative images obtained from filoquant for quantification of number and length of filopodia upon overexpression of Arp3 or Mrj in the HTT Q138 background.
Video showing time lapse imaging of a hemocyte expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals under HTT Q138 +Arp2/3 condition.
Video showing time lapse imaging of hemocyte expressing Lifeact tagged with GFP over 5 min at 5 s intervals under HTT Q138 +Arp2/3 condition.
Video showing time lapse imaging of a hemocyte expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals under HTT Q138 +Hip1 condition.
Video showing time lapse imaging of a hemocyte expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals under HTT Q138 +Mrj condition.
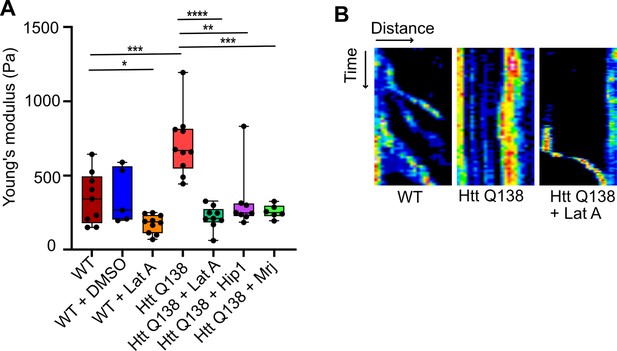
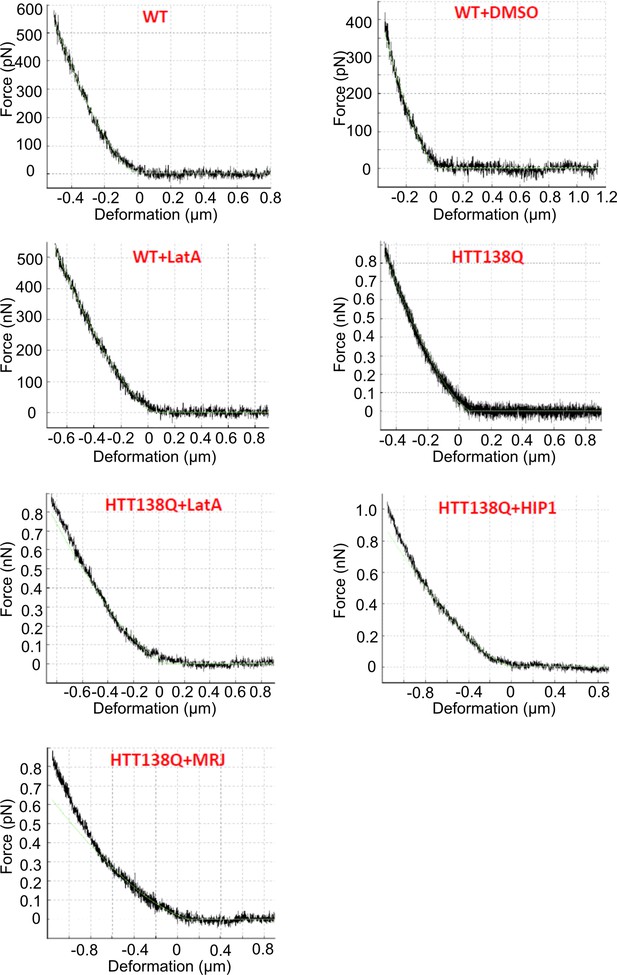
HTT Q138 expressing cells show increased cellular stiffness.
(A) Box plot showing Young’s modulus of WT, DMSO, WT +Lat A, HTT Q138, HTT Q138 +Lat A, HTT Q138 +Hip1 and HTT Q138 +Mrj cells. Number of cells: WT = 9; WT +DMSO = 5; HTT Q138=10; WT +Lat A=10; HTT Q138 +Lat A=9; HTT Q138 +Mrj = 6; HTT Q138 +Hip1=7. p-value = 0.0350, p-value = 0.0044 p-value <0.005, ****, p-value <0.0001 (Mann-Whitney test). (B) Kymographs showing movement of CCSs by live cell imaging of clathrin light chain tagged with GFP upon transient treatment with Lat A.
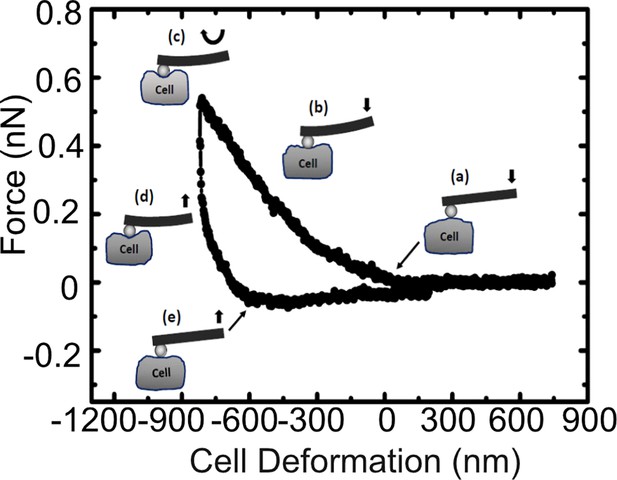
A representative force curve obtained on wild type cells.
The points (a), (b), (c), (d), and (e) represent the conditions at various phases of cell deformations while the bead indents on the cell, with the bead leaving the cell surface at point (e), compared to the point at which it makes contact while approaching (a).
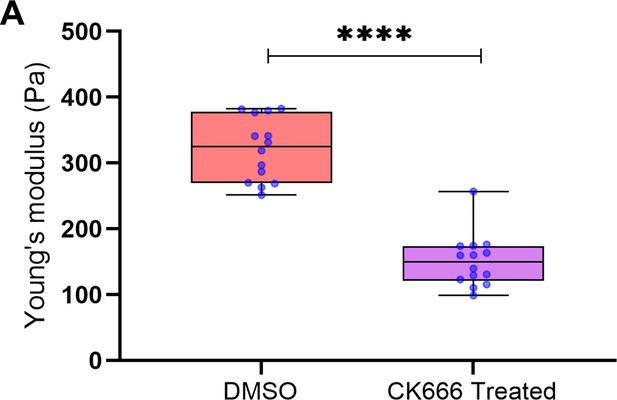
Box plot showing reduction in stiffness of cells upon treatment with CK666, P-value <0.0001 (Mann-Whitney test).
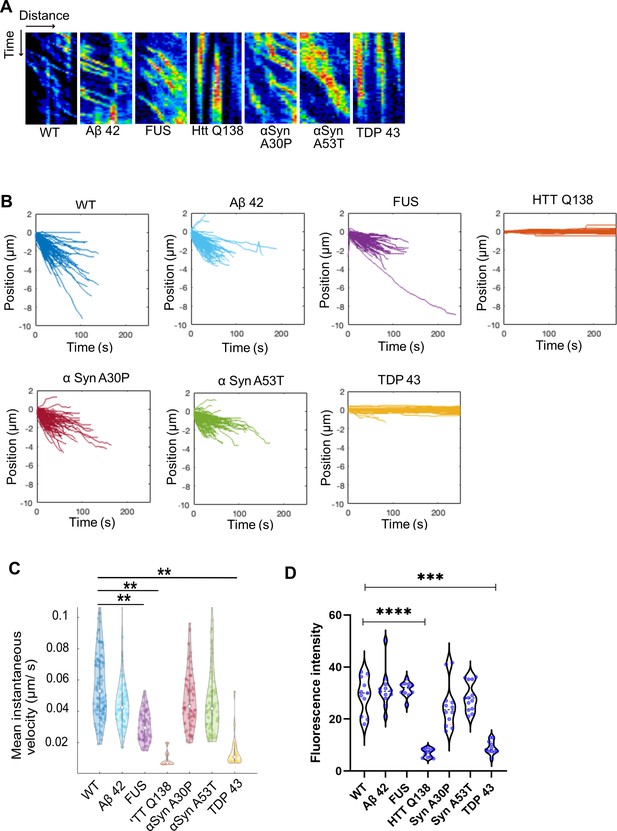
Cells expressing pathogenic TDP-43 show deficits in CCS movement.
(A) Kymographs showing the movement of CCSs from wild type hemocytes, or in the presence of the indicated proteins. (B) Position vs. time plots of CCSs in WT hemocytes and hemocytes expressing aggregating proteins. The positions of CCSs with respect to the cell centre are plotted. Negative positions indicate movement towards the cell centre. The total number of vesicles used for quantitation from ten cells are as follows: WT – 117; Aβ42–2 x – 105; FUSR521C – 83; HTT Q138 – 103; αSynA30P – 91; αSynA53T - 95 and TDP 43–106. (C) Violin plots of mean instantaneous velocities of CCSs in WT hemocytes and hemocytes expressing aggregating proteins. Asterisks represent a significant difference from WT (p<0.05, Kruskal Wallis test for non-parametric data). (D) Quantification of fluorescence intensity of internalized mBSA in hemocytes expressing the indicated aggregating proteins n=10. Statistical significance was determined using Kruskal-Wallis test followed by post hoc Dunn’s multiple comparison test. For WT vs HTT Q138 p-value <0.0001, **** and for WT vs TDP 43 p value = 0.0005 ***.
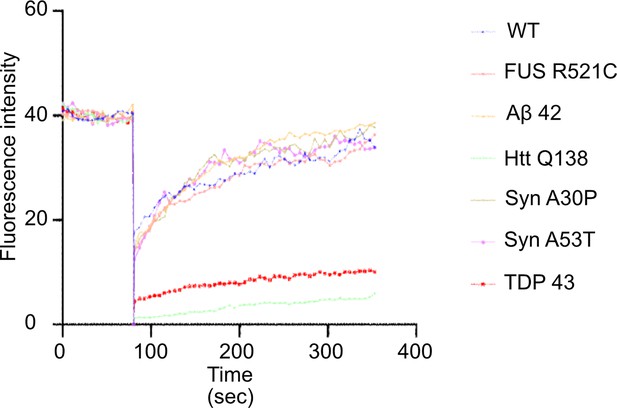
Graph showing fluorescence intensity of CLC GFP upon FRAP of individual CCSs.
Y-axis depicts GFP intensity and X-axis represents time in seconds. Only partial recovery of CLC GFP is seen in presence of HTT Q138 and TDP-43 whereas recovery of CLC GFP in other aggregate-containing cells is comparable to WT cells.
Video showing time lapse imaging of a hemocyte containing Aβ42 aggregates expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing FUS R521C aggregates expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing α-SynA30P aggregates expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing α-SynA53T aggregates expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing TDP-43 aggregates expressing Clathrin light chain tagged with GFP over 5 min at 5 s intervals.
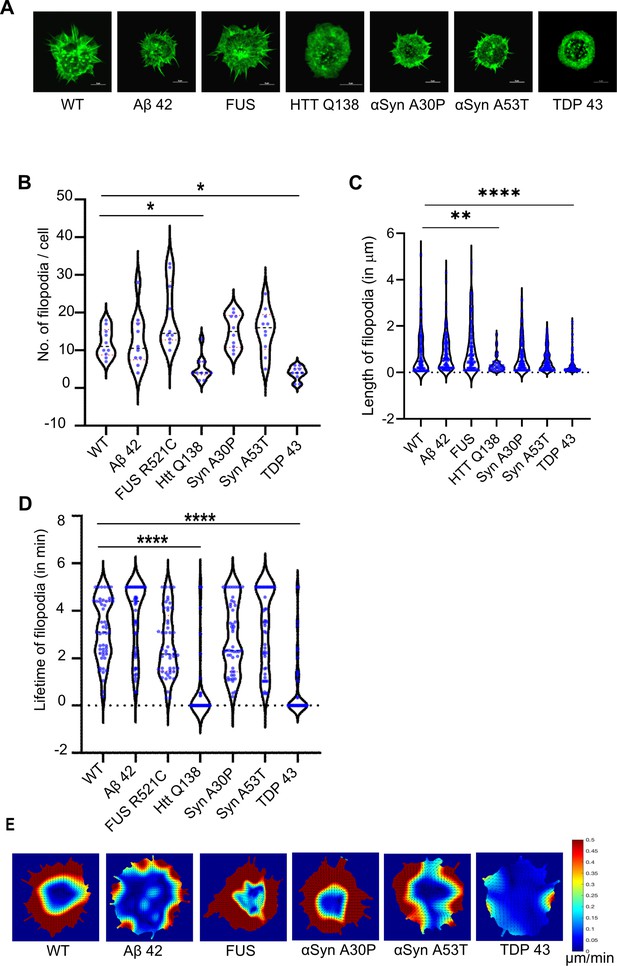
Cells expressing pathogenic TDP-43 show altered Actin dynamics.
(A) Representative micrographs showing the organization of actin marked by Lifeact-GFP in hemocytes. Scale bar 5 µm. (B) Graph showing the quantification of the number of filopodia per cell (n=10 cells for each condition). Statistical significance was determined using Kruskal-Wallis test followed by post hoc Dunn’s multiple comparison test. For WT vs HTT Q138 p-value = 0.0448,* and for WT vs TDP43 p-value = 0.0135, *. (C) Graph shows the quantification of length of filopodia in micrometres (n=10 cells for each condition). Statistical significance was determined using Kruskal-Wallis test followed by post hoc Dunn’s multiple comparison test. For WT vs HTT Q138 p-value = 0.0043, ** and for WT vs TDP 43 p value <0.0001, ****. The number and length of filopodia were quantified using the Filoquant plugin in ImageJ. (D) Violin plots of the lifetime of filopodia in cells expressing Aβ42–2 x, FUSR521C, αSynA30P, αSynA53T, HTT Q138, TDP-43 compared to WT cells. Kruskal-Wallis test followed by post hoc Dunn’s multiple comparison was performed to calculate the p value. (p-value <0.0001). (E) PIV analysis performed on LifeAct-GFP-expressing cells in the presence of the indicated pathogenic aggregating proteins to highlight the direction and magnitude of actin flow.
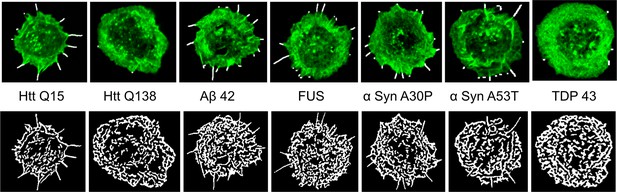
Micrograph showing representative images obtained from Filoquant for quantification of number and length of filopodia in presence of the indicated mutant protein.
Video showing time lapse imaging of a hemocyte containing Aβ42 aggregates expressing Lifeact tagged with GFP over 5 min at 5 second intervals.
Video showing time lapse imaging of a hemocyte containing FUS R521C aggregates expressing Lifeact tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing α-SynA30P aggregates expressing Lifeact tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing α-SynA53T aggregates expressing Lifeact tagged with GFP over 5 min at 5 s intervals.
Video showing time lapse imaging of a hemocyte containing TDP 43 aggregates expressing Lifeact tagged with GFP over 5 min at 5 s intervals.
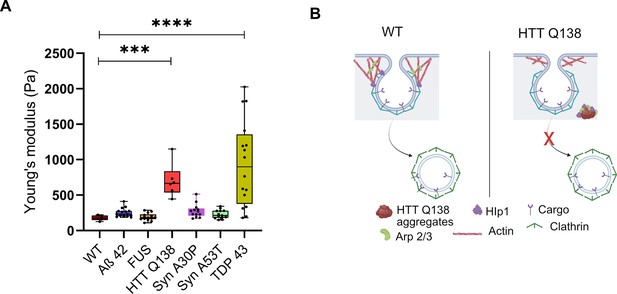
TDP-43 aggregates result in the alteration of cellular physical properties.
(A) Box plot showing the Young’s modulus of all the indicated cell types. AFM was performed on 10 cells of each cell type ***, p-value = 0.0005 and ****, p-value <0.0001. Statistical significance was determined using Kruskal-Wallis test followed by post-hoc Dunn’s multiple comparisons. (B) Model showing alteration in actin organization and clathrin-mediated endocytosis in the presence of Huntingtin aggregates due to sequestration of Hip1 and components of the Arp2/3 complex.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/98363/elife-98363-mdarchecklist1-v2.docx
-
Supplementary file 1
Table showing the pixel and frame rate information for each cell type.
- https://cdn.elifesciences.org/articles/98363/elife-98363-supp1-v2.docx
-
Source data 1
Source data for the figures included in the manuscript.
- https://cdn.elifesciences.org/articles/98363/elife-98363-data1-v2.xlsx
-
Source data 2
Source data for PIV plots included in this manuscript.
- https://cdn.elifesciences.org/articles/98363/elife-98363-data2-v2.zip