Conservation of the cooling agent binding pocket within the TRPM subfamily
Figures
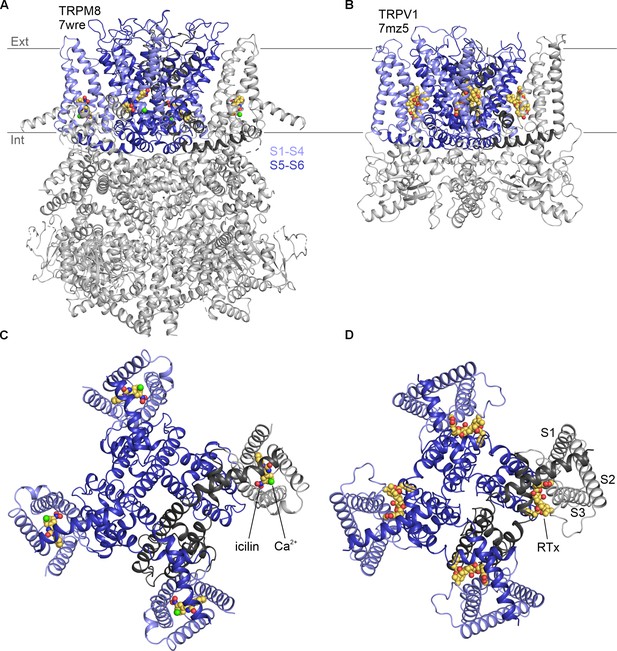
Structures of vanilloid bound to TRPV1 and cooling agent bound to TRPM8.
Side views of (A) TRPM8 bound to icilin (CPK) and Ca2+ (green sphere) and (B) TRPV1 bound to RTx (CPK). Intracellular views of (C) TRPM8 bound to icilin and Ca2+ and (D) TRPV1 bound to RTx.
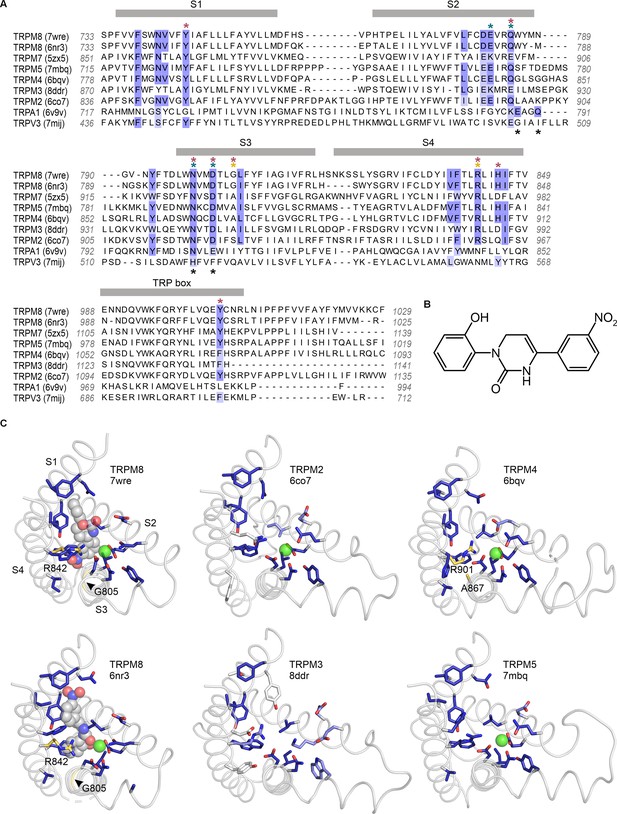
Sequence and structure conservation of the icilin binding pocket in TRPM and TRPA channels.
(A) Structure-based sequence alignment of S1–S4 peripheral domains and transient receptor potential (TRP) helix of selected TRP channel structures, with residues contributing to the icilin binding pocket in TRPM8 structures (7wre and 6nr3) highlighted in blue. The equivalent residues in other channels are colored according to the alignment quality score calculated from multiple sequence alignments, where highly conserved residues are color blue and poorly conserved residues are colored in white. Alignment quality score calculated in Jalview based on BLOSUM 62 scores (Henikoff and Henikoff, 1992). Teal asterisks indicate Ca2+-coordinating residues in structures of TRPM channels. Black asterisks indicated Ca2+-coordinating residues in TRPA1. Red asterisks indicated residues where mutation influence cooling agent sensitivity in TRPM8. Gold asterisks indicate residues mutated in the present study. (B) Chemical structure of icilin. (C) S1–S4 residues contributing to the icilin binding pocket in TRPM8 structures (7wre and 6nr3) are shown as blue licorice, viewed from the intracellular side of the membrane as in Figure 1C, with the TRP box omitted for clarity. Cooling agent binding pocket mutations used in the present study are shown with carbon atoms colored gold and labeled in TRPM8 and TRPM4, and the equivalent residues in other channels are colored based on the alignment quality score, as in panel A. 7wre is mTRPM8, 6nr3 is faTRPM8 containing the A805G mutation, 6co7 is Nematostella vectensis TRPM2, 8ddr is mTRPM3, 6bqv is hTRPM4, and 7mbq is zebra fish TRPM5. Sequence identity between residues within the icilin binding pocket of TRPM8 and corresponding residues in the other TRP channel is as follows: TRPM5 (94%), TRPM4 (89%), TRPM2 (78%), TRPM3 and TRPM7 (44%), TRPA1 (22%), and TRPV3 (11%).
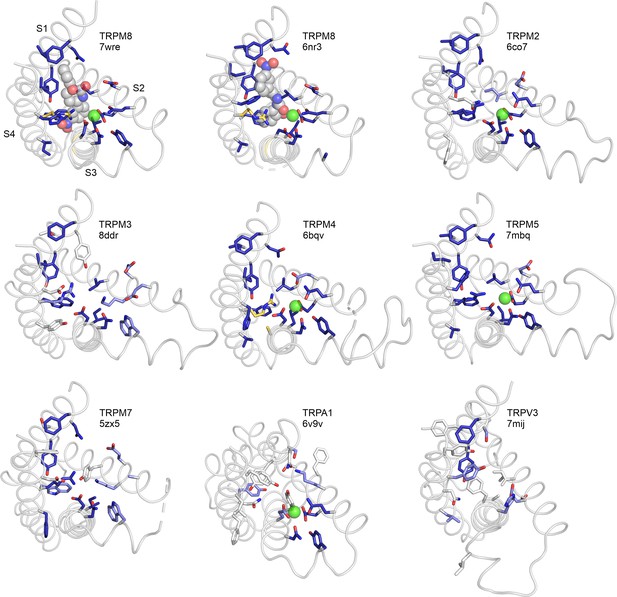
S1–S4 residues contributing to the icilin binding pocket in TRPM8 structures 7wre and 6nr3 are shown as blue licorice, viewed from the intracellular side of the membrane, with the transient receptor potential (TRP) box omitted for clarity.
Cooling agent binding pocket mutations used in the present study are shown with carbon atoms colored gold and labeled in TRPM8 and TRPM4, and the equivalent residues in other channels are colored based on the alignment quality score, as in Figure 2A.
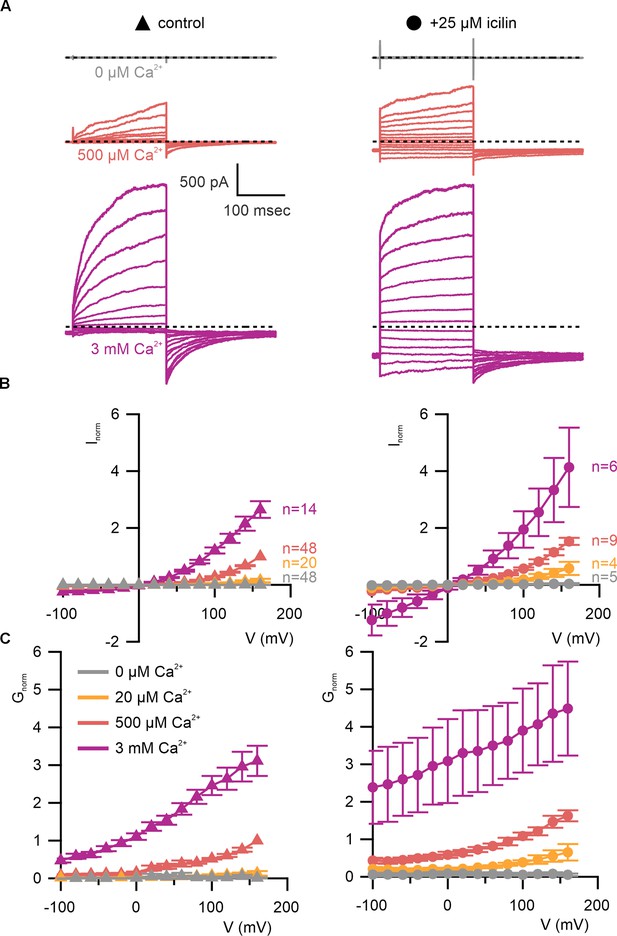
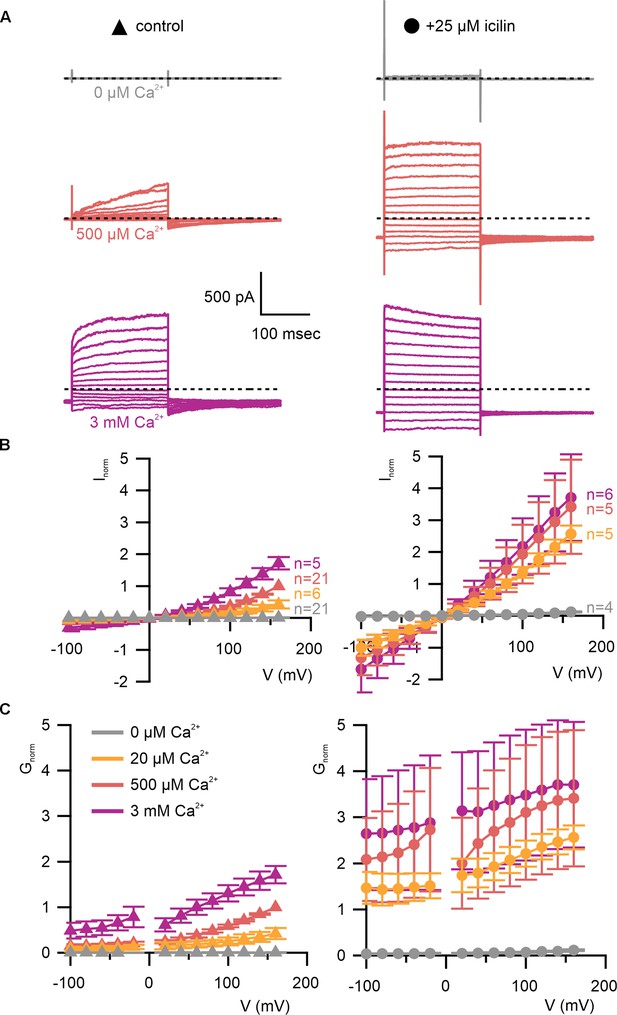
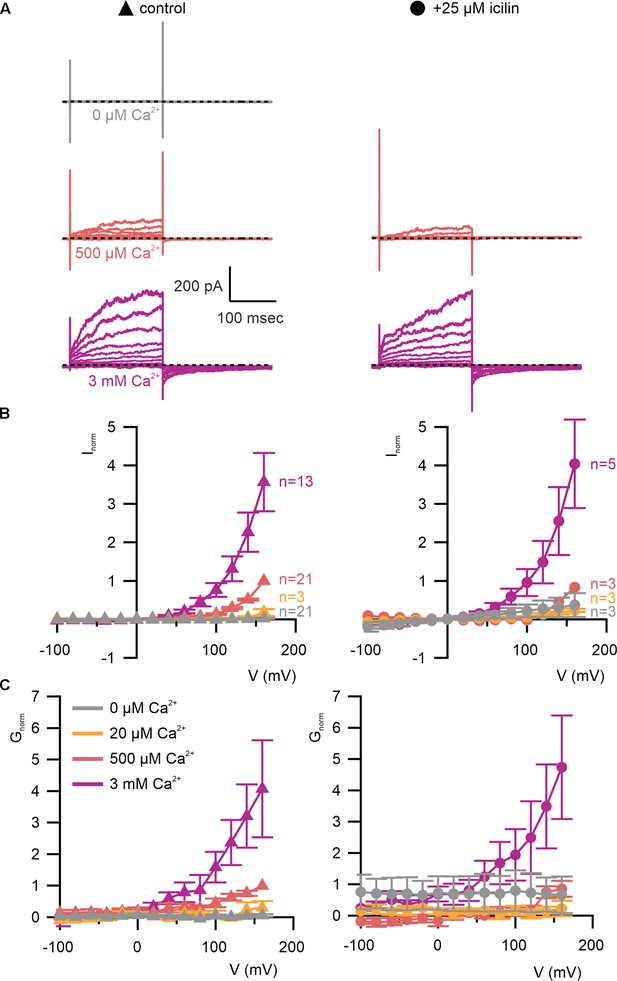
WT TRPM4 is sensitive to intracellular Ca2+, voltage, and icilin.
(A) Sample current families obtained using a holding voltage of −60 mV with 200 ms steps to voltages between −100 and +160 mV (Δ 20 mV) before returning to −60 mV. Control traces in the left column were obtained with TRPM4 in the absence of icilin and the presence of the labeled Ca2+ concentrations, and traces in the right column were obtained in the presence of 25 µM icilin and the labeled Ca2+ concentrations. (B) Normalized I–V and (C) normalized G–V plots for populations of cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Conductance values were obtained from tail current measurements. For each cell, values are normalized to the steady-state current or conductance at +160 mV in the presence of 500 µM Ca2+. Error bars indicate standard error of the mean.
-
Figure 3—source data 1
Excel file with electrophysiology data for Figure 3B.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Excel file with electrophysiology data for Figure 3C.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig3-data2-v1.xlsx
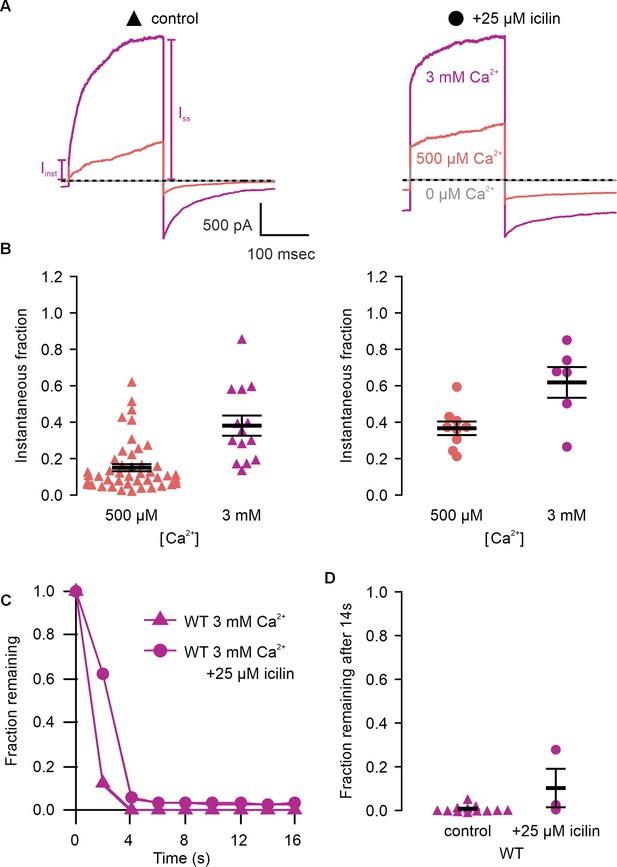
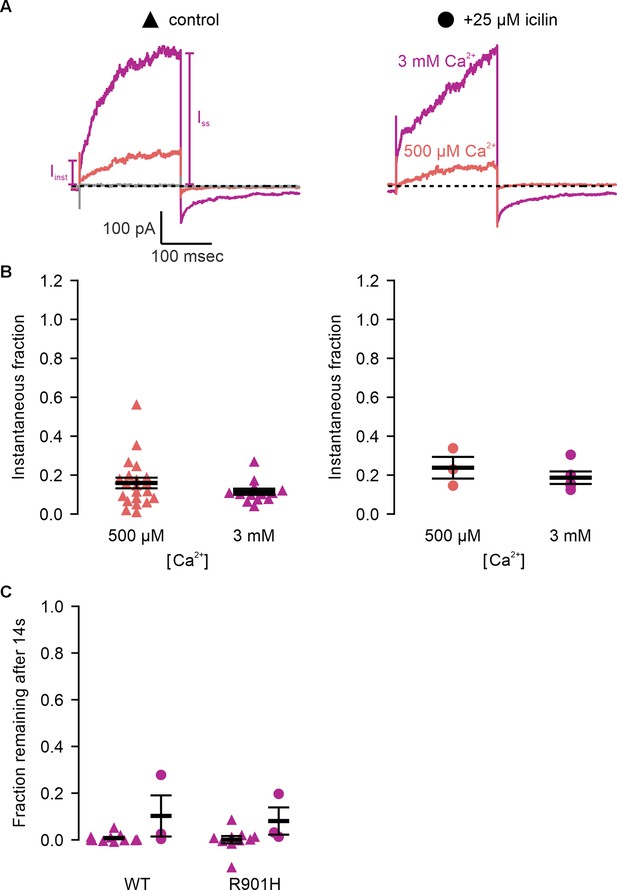
Icilin modulates voltage-dependent activation and closure kinetics of TRPM4.
(A) Sample current traces illustrating the fraction of current that activates rapidly (Iinst) compared to the steady-state current at the end of the pulse (ISS). The pulse protocols used a holding voltage of −60 mV with 200 ms steps to +160 mV in the presence of varying concentrations of intracellular Ca2+. Traces were obtained in the absence (left) or presence (right) of 25 µM icilin. (B) Instantaneous fraction of current (Iinst/ISS) calculated using voltage steps to +160 mV at various concentrations of intracellular Ca2+ for individual cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Error bars indicate standard error of the mean. (C) Fraction of current remaining after application of 3 mM Ca2+ alone (triangles) or both 3 mM Ca2+ and 25 µM icilin (squares) for WT TRPM4. Currents were elicited by voltage steps from −100 to +100 mV. (D) Fraction of current remaining 14 s after removal of 3 mM Ca2+ alone (triangles) or both 3 mM Ca2+ and 25 µM icilin (squares) for WT TRPM4. Currents were elicited by voltage steps from −100 to +160 mV.
-
Figure 4—source data 1
Excel file with electrophysiology data for Figure 4B–D.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig4-data1-v1.xlsx
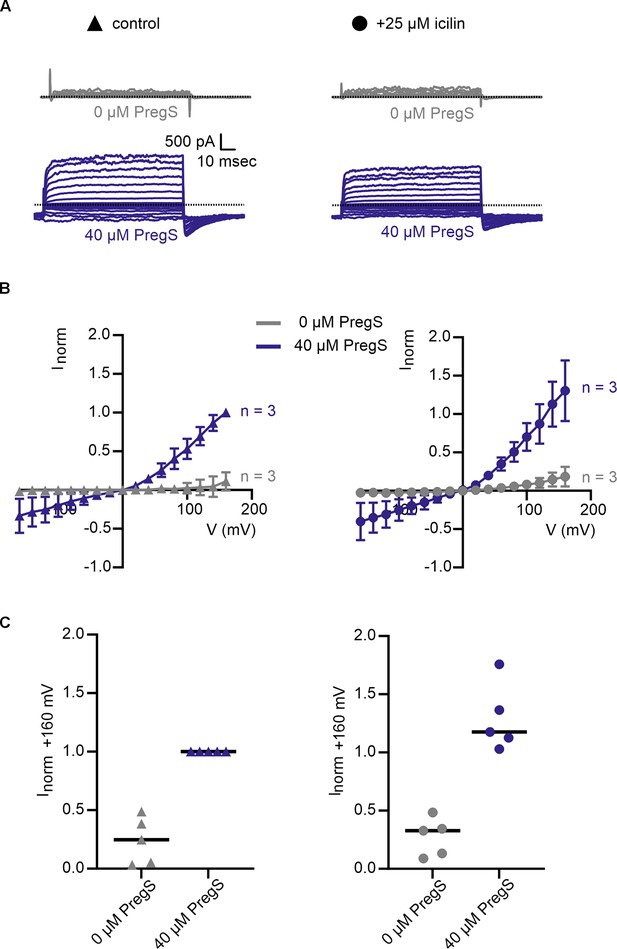
Icilin does not modulate voltage-dependent activation of TRPM3α2.
(A) Sample current families obtained using a holding voltage of −60 mV with 50 ms prepulses to −150 mV before 100 ms steps to voltages between −160 and +160 mV (Δ 20 mV), and then returning to −60 mV. Control traces in the left column were obtained with TRPM3α2 in the absence of icilin (left, triangles), and traces in the right column were obtained in the presence of 25 µM icilin (right, circles). Activation of TRPM3 current is achieved with 40 µM pregnenolone sulfate (PregS, navy). (B) Corresponding normalized I–V relations and (C) plots of normalized current evoked at +160 mV in populations of cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Absolute values were obtained from steady-state current measurements. For each cell, values are normalized to the absolute current at +160 mV in the presence of 40 µM PregS. Error bars indicate standard error of the mean.
-
Figure 4—figure supplement 1—source data 1
Excel file with electrophysiology data for Figure 4—figure supplement 1A.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig4-figsupp1-data1-v1.xlsx
-
Figure 4—figure supplement 1—source data 2
Excel file with electrophysiology data for Figure 4—figure supplement 1B, C.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig4-figsupp1-data2-v1.xlsx
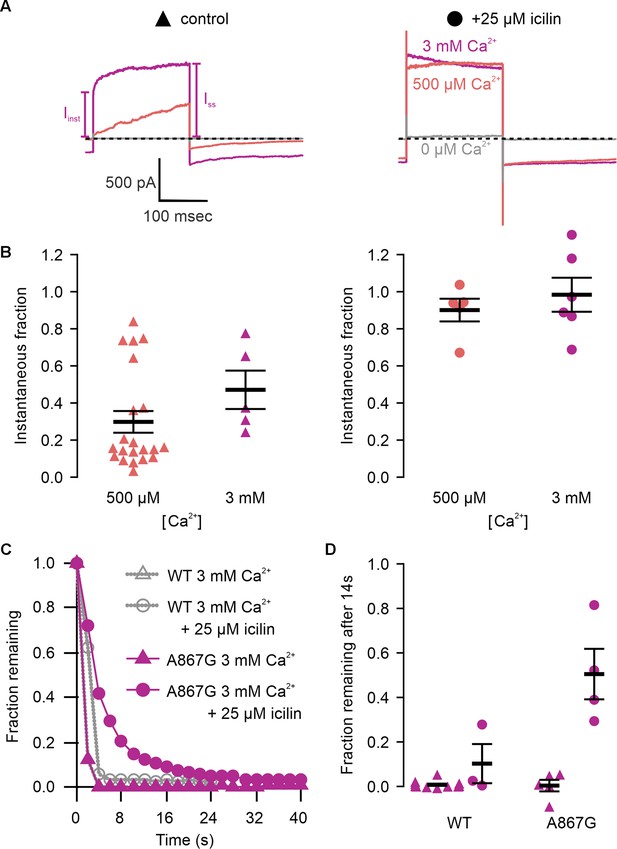
A867G mutant TRPM4 retains sensitivity to Ca2+ and voltage, but has enhanced sensitivity to icilin.
(A) Sample current families obtained using a holding voltage of −60 mV with 200 ms steps to voltages between −100 and +160 mV (Δ 20 mV) before returning to −60 mV. Control traces in the left column were obtained with A867G mTRPM4 in the absence of icilin and the presence of the labeled Ca2+ concentrations, and traces in the right column were obtained in the presence of 25 µM icilin and the labeled Ca2+ concentrations. (B) Normalized I–V and (C) normalized G–V plots for populations of cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Conductance values were calculated from steady-state currents. For each cell, values are normalized to the steady-state current or conductance at +160 mV in the presence of 500 µM Ca2+. Error bars indicate standard error of the mean.
-
Figure 5—source data 1
Excel file with electrophysiology data for Figure 5B.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Excel file with electrophysiology data for Figure 5C.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig5-data2-v1.xlsx
Icilin modulation of TRPM4 is enhanced in the A867G mutant.
(A) Sample current traces illustrating the fraction of current that activates rapidly (Iinst) compared to the steady-state current at the end of the pulse (ISS). The pulse protocols used a holding voltage of −60 mV with 200 ms steps to +160 mV in the presence of varying concentrations of intracellular Ca2+. Traces were obtained in the absence (left) or presence (right) of 25 µM icilin. (B) Instantaneous fraction of current (Iinst/ISS) calculated using +160 mV voltage steps at various concentrations of intracellular Ca2+ for individual cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Error bars indicate standard error of the mean. (C) Fraction of current remaining after application of 3 mM Ca2+ alone (triangles) or both 3 mM Ca2+ and 25 µM icilin (squares) for WT TRPM4 (gray) or A867G TRPM4 (purple). Currents were elicited by voltage steps from −100 to +100 mV. (D) Fraction of current remaining 14 s after removal of 3 mM Ca2+ alone (triangles) or both 3 mM Ca2+ and 25 µM icilin (squares) for WT (left) or A867G TRPM4 (right). Currents were elicited by voltage steps from −100 to +160 mV.
-
Figure 6—source data 1
Excel file with electrophysiology data for Figure 6B–D.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig6-data1-v1.xlsx
R901H mutant TRPM4 is sensitive to intracellular Ca2+ and voltage, but icilin does not promote opening.
(A) Sample current families obtained using a holding voltage of −60 mV with 200 ms steps to voltages between −100 and +160 mV (Δ 20 mV) before returning to −60 mV. Control traces in the left column were obtained with R901H mTRPM4 in the absence of icilin and the presence of the labeled Ca2+ concentrations, and traces in the right column were obtained in the presence of 25 µM icilin and the labeled Ca2+ concentrations. For the cell shown, current families were not obtained in the presence of icilin and the absence of Ca2+. (B) Normalized I–V and (C) normalized G–V plots for populations of cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Conductance values were obtained from tail current measurements. For each cell, values are normalized to the steady-state current or conductance at +160 mV in the presence of 500 µM Ca2+. Error bars indicate standard error of the mean.
-
Figure 7—source data 1
Excel file with electrophysiology data for Figure 7B.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig7-data1-v1.xlsx
-
Figure 7—source data 2
Excel file with electrophysiology data for Figure 7C.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig7-data2-v1.xlsx
Icilin modulation of the voltage-dependent activation of TRPM4 is disrupted in the R901H mutant.
(A) Sample current traces illustrating the fraction of current that activates rapidly (Iinst) compared to the steady-state current at the end of the pulse (ISS). The pulse protocols used a holding voltage of −60 mV with 200 ms steps to +160 mV in the presence of varying concentrations of intracellular Ca2+. Traces were obtained in the absence (left) or presence (right) of 25 µM icilin. For the cell shown, current families were not obtained in the presence of icilin and absence of Ca2+. (B) Instantaneous fraction of current (Iinst/ISS) calculated using +160 mV voltage steps at various concentrations of intracellular Ca2+ for individual cells in the absence (left, triangles) or presence (right, circles) of 25 µM icilin. Error bars indicate standard error of the mean. (C) Fraction of current remaining after 14 s of 0 mM Ca2+ wash, following removal of 3 mM Ca2+ alone (triangles) or both 3 mM Ca2+ and 25 µM icilin (squares) for WT (left) or R901H TRPM4 (right). Currents were measured between −100 and +160 mV (Δ20 mV), but only +160 mV current fractions are shown.
-
Figure 8—source data 1
Excel file with electrophysiology data for Figure 8B, C.
- https://cdn.elifesciences.org/articles/99643/elife-99643-fig8-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HEK293 | ATCC | CRL-1573 ATCC Cat# PTA-4488, RRID:CVCL_0045 | |
| Transfected construct (Mus musculus) | mTRPM4b (plasmid) | Received from Youxing Jiang | pEGFP-N1 vector | |
| Transfected construct (Mus musculus) | mTRPM3α2 (plasmid) | Received from Thomas Voets | pCAGGS/IRES-GFP vector | |
| Commercial assay or kit | FuGENE6 Transfection Reagent | Promega | E269 | |
| Commercial assay or kit | QuikChange Lightning | Agilent Technologies | 21051 | |
| Chemical compound, drug | Icilin | Sigma | CAS 36945-98-9 | Source # 0000088284 Source # 0000141718 |
| Chemical compound, drug | Pregnenolone sulfate | Sigma | P162-25MG | Lot # MKCQ8813 |
| Software, algorithm | IgorPro | WaveMetrics | RRID:SCR_000325 | Data analysis |
| Software, algorithm | Python programming language | RRID:SCR_008394 | Data analysis | |
| Software, algorithm | SciPy | RRID:SCR_008058 | Data analysis | |
| Software, algorithm | Matplotlib | RRID:SCR_008624 | Data visualization | |
| Software, algorithm | seaborn | RRID:SCR_018132 | Data visualization | |
| Software, algorithm | PyMOL | Schrödinger | RRID:SCR_000305 | Structure visualization |
| Software, algorithm | PoseFilter | Subha Kalyaanamoorthy; https://doi.org/10.1093/bioinformatics/btab188 | Data analysis PyMOL Plugin | |
| Software, algorithm | pClamp 11 | Molecular Devices | RRID:SCR_011323 | Acquisition patch clamp data |
| Software, algorithm | JalView | RRID:SCR_006459 | Sequence alignment visualization | |
| Software, algorithm | Fr-TM-Align | Pandit & Skolnick; https://doi.org/10.1186/1471-2105-9-531 | Data analysis |





