Microtubules: May I check your cap?
Microtubules are hollow cylindrical polymers that have important roles in chromosome segregation, organelle transport and other processes inside cells. Microtubules are built from protein subunits called αβ-tubulin and abruptly switch between growing and shrinking. This switching property is known as "dynamic instability", and has captivated scientists since it was discovered over 30 years ago (Horio and Hotani, 1986; Mitchison and Kirschner, 1984).
The switch from the growing state to the shrinking state is known as catastrophe, and is essential for microtubules to work correctly. Catastrophe occurs when the microtubule loses its stabilizing cap, which is a biochemically distinct region near the growing end. Despite substantial efforts, both the size of this stabilizing cap and its relationship to microtubule growth rates have remained obscure. Now, in eLife, Thomas Surrey and co-workers at the Francis Crick Institute and the London Centre for Nanotechnology – including Christian Duellberg as first author – use state-of-the-art methods to resolve these longstanding conundrums (Figure 1; Duellberg et al., 2016).
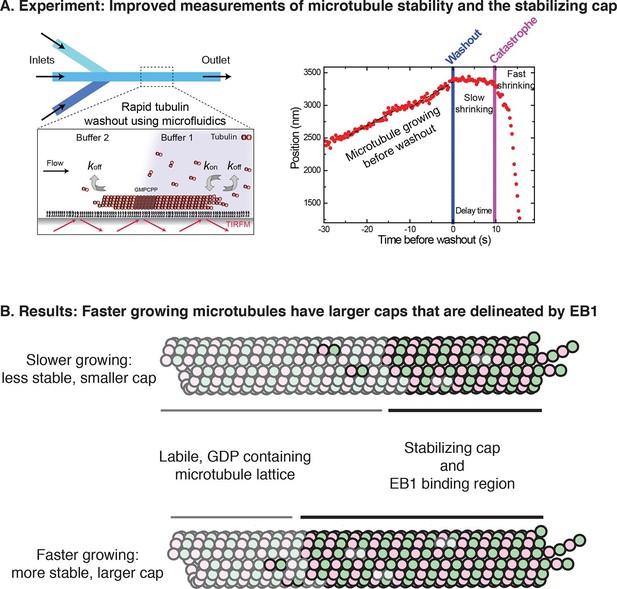
A modernized form of a classic technique enables the growth and stabilization of microtubules to be studied.
(A) Left: Duellberg et al. used microfluidics to abruptly stop microtubule growth via the "washout" approach. Right: Sample data showing microtubule length versus time. Before washout, the microtubule grows steadily; after washout, it shrinks slowly for a time; and after catastrophe, it shrinks rapidly (Panel adapted from Figures 1A and 2A, Duellberg et al.). (B) Duellberg et al. observed correlations between the microtubule growth rate and the size of the stabilizing cap, which consists of GTP-bound αβ-tubulin subunits (indicated by the non-faded circles). The caps are marked by EB1 proteins (not shown explicitly).
In a simple sense, microtubule catastrophe results from a race between two competing processes. Microtubules grow by capturing αβ-tubulin subunits that are bound to a molecule called GTP. However, shortly after a new subunit is added to the polymer, its GTP molecule is hydrolyzed: this destabilizes the polymer and provides the driving force for catastrophe.
The time interval between the addition of the subunit and the hydrolysis of the GTP should create a cap that protects the growing microtubule against catastrophe. This simple view predicts that faster growing microtubules should have larger caps. Over the years, however, experiments to test this prediction have yielded conflicting results (Caplow and Shanks, 1996; Schek et al., 2007), resulting in a proliferation of different models that attempt to describe microtubule stabilization (reviewed in Bowne-Anderson et al., 2013).
“Washout” experiments are a classic way to measure microtubule stability and estimate cap size by suddenly diluting the solution around growing microtubules to stop their growth (Walker et al., 1991). After washout, the microtubule shrinks slowly for a short period of time before it begins to shrink rapidly. The time delay before rapid shrinking occurs is related to the size of the stabilizing cap. Using this approach, a classic early paper failed to find a relationship between the rate at which microtubules grow and their stability (Walker et al., 1991).
Duellberg et al. have now revitalized this washout approach by developing a new microfluidics-based method that enables much faster dilution (Figure 1A). This method also incorporates high-precision microtubule end tracking and averaging techniques previously developed by Surrey and co-workers (Maurer et al., 2014).
The new approach allowed Duellberg et al. to demonstrate that microtubules that are growing faster at the time of dilution experience a longer delay before they begin to rapidly shrink (Figure 1B). The cap size could also be estimated from the length of the slow-shrinking phase. Thus, microtubule growth rate and microtubule stability are correlated.
To provide more direct insight into the size of the cap and how it is lost, Duellberg et al. turned to the EB1 family of microtubule regulatory proteins. These proteins form ‘comets’ by binding to an extended region near the microtubule end (Bieling et al., 2007), and are thought to recognize unique structural features of the stabilizing cap (Zhang et al., 2015).
To determine whether microtubule stability is related to the size of EB comets, Duellberg et al. simultaneously monitored microtubule ends and fluorescently tagged EB proteins. They observed that faster growing microtubules recruited more EB protein at the growing end (as shown by more intense fluorescent ‘comets’). The number of high-affinity EB binding sites decreased exponentially after washout, and rapid shrinking began when the density of EB binding sites was reduced to about 20% of its maximal value.
Consistent with these findings, microtubules with more EB binding sites (brighter comets) at the time of dilution exhibited a longer time delay before rapid shrinking began. This time delay can also be predicted if the rates of three processes are known: microtubule growth, slow shrinkage and EB binding site loss.
By demonstrating that EB proteins label the cap region and therefore provide a way to visualize it, and by resolving a longstanding ambiguity about the relationship between the cap and microtubule growth rate, Duellberg et al. advance our understanding of microtubule stabilization. However, we cannot currently explain Duellberg et al.’s findings in terms of the conformations and biochemical properties of individual αβ-tubulin subunits. More generally, microtubules normally undergo catastrophe without the sudden dilution used by Duellberg et al.: defining the mechanisms that normally trigger catastrophe therefore remains another important challenge.
The high-quality data that Duellberg et al. report set a new standard for studies of other microtubule regulatory proteins. Their pioneering quantitative analyses will undoubtedly contribute to future advances in the understanding of dynamic instability and its regulation.
References
-
Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubulesMolecular Biology of the Cell 7:663–675.https://doi.org/10.1091/mbc.7.4.663.
-
Microtubule assembly dynamics at the nanoscaleCurrent Biology 17:1445–1455.https://doi.org/10.1016/j.cub.2007.07.011
Article and author information
Author details
Publication history
- Version of Record published: April 6, 2016 (version 1)
Copyright
© 2016, Geyer et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,303
- views
-
- 114
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Structural Biology and Molecular Biophysics
The type II class of RAF inhibitors currently in clinical trials paradoxically activate BRAF at subsaturating concentrations. Activation is mediated by induction of BRAF dimers, but why activation rather than inhibition occurs remains unclear. Using biophysical methods tracking BRAF dimerization and conformation, we built an allosteric model of inhibitor-induced dimerization that resolves the allosteric contributions of inhibitor binding to the two active sites of the dimer, revealing key differences between type I and type II RAF inhibitors. For type II inhibitors the allosteric coupling between inhibitor binding and BRAF dimerization is distributed asymmetrically across the two dimer binding sites, with binding to the first site dominating the allostery. This asymmetry results in efficient and selective induction of dimers with one inhibited and one catalytically active subunit. Our allosteric models quantitatively account for paradoxical activation data measured for 11 RAF inhibitors. Unlike type II inhibitors, type I inhibitors lack allosteric asymmetry and do not activate BRAF homodimers. Finally, NMR data reveal that BRAF homodimers are dynamically asymmetric with only one of the subunits locked in the active αC-in state. This provides a structural mechanism for how binding of only a single αC-in inhibitor molecule can induce potent BRAF dimerization and activation.
-
- Structural Biology and Molecular Biophysics
We integrate evolutionary predictions based on the neutral theory of molecular evolution with protein dynamics to generate mechanistic insight into the molecular adaptations of the SARS-COV-2 spike (S) protein. With this approach, we first identified candidate adaptive polymorphisms (CAPs) of the SARS-CoV-2 S protein and assessed the impact of these CAPs through dynamics analysis. Not only have we found that CAPs frequently overlap with well-known functional sites, but also, using several different dynamics-based metrics, we reveal the critical allosteric interplay between SARS-CoV-2 CAPs and the S protein binding sites with the human ACE2 (hACE2) protein. CAPs interact far differently with the hACE2 binding site residues in the open conformation of the S protein compared to the closed form. In particular, the CAP sites control the dynamics of binding residues in the open state, suggesting an allosteric control of hACE2 binding. We also explored the characteristic mutations of different SARS-CoV-2 strains to find dynamic hallmarks and potential effects of future mutations. Our analyses reveal that Delta strain-specific variants have non-additive (i.e., epistatic) interactions with CAP sites, whereas the less pathogenic Omicron strains have mostly additive mutations. Finally, our dynamics-based analysis suggests that the novel mutations observed in the Omicron strain epistatically interact with the CAP sites to help escape antibody binding.





