Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system
Abstract
Axon guidance is proposed to act through a combination of long- and short-range attractive and repulsive cues. The ligand-receptor pair, Netrin (Net) and Frazzled (Fra) (DCC, Deleted in Colorectal Cancer, in vertebrates), is recognized as the prototypical effector of chemoattraction, with roles in both long- and short-range guidance. In the Drosophila visual system, R8 photoreceptor growth cones were shown to require Net-Fra to reach their target, the peak of a Net gradient. Using live imaging, we show, however, that R8 growth cones reach and recognize their target without Net, Fra, or Trim9, a conserved binding partner of Fra, but do not remain attached to it. Thus, despite the graded ligand distribution along the guidance path, Net-Fra is not used for chemoattraction. Based on findings in other systems, we propose that adhesion to substrate-bound Net underlies both long- and short-range Net-Fra-dependent guidance in vivo, thereby eroding the distinction between them.
https://doi.org/10.7554/eLife.20762.001eLife digest
The brain of the fruit fly contains hundreds of thousands of neurons, while the human brain contains more than 80 billion. Each of these consists of a cell body that bears an array of branches called dendrites, plus a single cable-like axon. During development, the neurons organize themselves into complex networks by forming connections with one another via their axons and dendrites. But it is not clear exactly how the correct connections form in the correct places.
As they grow out, axons rely on specialized moving structures at their tips – known as growth cones – to probe their environment in search of attractive and repulsive chemical signals released by other cells. When sensors on the surface of growth cones detect a target signal, they initiate processes that cause the growth cone to expand or collapse. This enables the axons to move towards or away from the signal, as appropriate. In all animals studied, proteins called DCC and Netrin form one of the best-known sensor-signal pairs. Growth cones bearing DCC sensors are thought to detect ‘wafting plumes’ or gradients of Netrin and then grow towards the Netrin source.
However, nobody had directly watched neurons respond to Netrin in a living intact animal. Using a type of microscope that can look deep into the developing fly brain, Akin and Zipursky have now followed the movement of growth cones on cells called R8 neurons in fruit fly pupae. Unexpectedly, Akin and Zipursky found that the growth cones of mutant flies that lack Netrin or Frazzled (the fruit fly version of DCC) navigate successfully to their intended destinations. Once there, however, the mutant growth cones were unable to attach to their targets.
Akin and Zipursky’s work is consistent with other observations in a number of animal and insect systems that suggest that Netrin may not attract growth cones via wafting plumes of signal. Instead, Netrin may form a sticky trail that helps growth cones to gain traction as they crawl towards or stick to their destinations. Further experiments are now needed to test whether other neurons in fruit flies and in different animals use Netrin in this way.
https://doi.org/10.7554/eLife.20762.002Introduction
Net, a secreted protein, and its receptor DCC together play a critical role in wiring the brain in both vertebrates and invertebrates. Net-DCC mediated axon guidance has been well characterized in the developing vertebrate spinal cord. In brief, Net expressed at the floor plate is proposed to diffuse to establish a decreasing ventral-to-dorsal gradient within the spinal cord (Kennedy et al., 2006; Serafini et al., 1996). This gradient, in turn, is proposed to promote the guidance of commissural neuron growth cones ventrally to the floor plate (Fazeli et al., 1997; Serafini et al., 1996). Classic in vitro studies using purified proteins and explant cultures also support a role for Net as a chemoattractant (de la Torre et al., 1997; Kennedy et al., 1994).
Net-DCC based axon guidance to the midline is an evolutionarily conserved mechanism in nervous system development. In C. elegans, loss of UNC-6 - UNC-40 (the C. elegans homologs of Net-DCC) signaling leads to dorsal displacement of axon tracts that are positioned ventrally in wild-type (Chan et al., 1996; Hedgecock et al., 1990; Ishii et al., 1992). And in Drosophila, Net and Frazzled (Fra, the Drosophila homolog of DCC) are required for axons to cross the midline of the embryonic ventral nerve cord (Harris et al., 1996; Kolodziej et al., 1996). Here, a number of neurons from each side of the midline send their axons contralaterally, creating the commissures of the ventral nerve cord. Net is expressed at the midline by glia and Net protein is found as a gradient that peaks at the midline. In Net or fra mutants, the commissures of the ventral nerve cord are missing or severely reduced, consistent with a loss of chemoattraction to the ligand source.
The spatial relationship between a Net-responsive growth cone and the source of Net in the fly visual system is similar to both the fly midline and vertebrate spinal cord. The fly visual system is modular, comprising some 750 columns. Different neuronal cell types, including R8, extend axons within each column, where they terminate in different layers. R8 growth cones reach their target, the M3 layer of the medulla neuropil, in two steps (Ting et al., 2005) (Figure 1a). They ‘park’ at a temporary target at the outer surface of the medulla, referred to as M0, and then, after a delay, extend to and terminate within the M3 layer. Salecker and colleagues demonstrated that R8 targeting to M3 requires Net-Fra signaling (Timofeev et al., 2012). R8s express Fra; Net is expressed by L3 growth cones within the M3 layer and is seen as a shallow gradient that stretches from M0 to M3, peaking sharply at M3 (Timofeev et al., 2012). R8 growth cones extend from M0 to M3 within a dense neuropil containing the processes of many different cell types. Without Fra or Net, many R8 terminals remain in M0 while others are stranded between M0 and M3 (Timofeev et al., 2012).
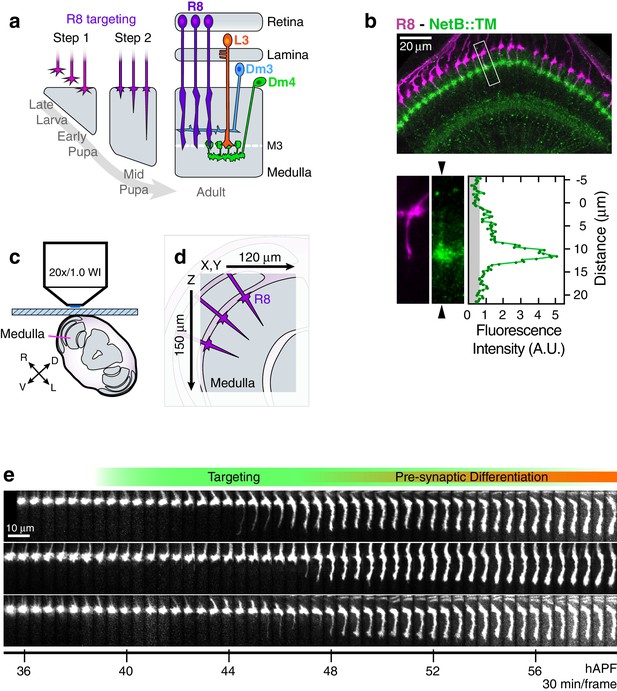
Live imaging of R8 growth cones in the developing fly brain.
(a) Schematic of R8 targeting. (b), Top panel: Confocal micrograph of the medulla at 45 hAPF in a fly expressing a membrane-tethered variant of NetB (NetB::TM) from the NetB genomic locus. R8s (magenta, myr::tdTOM) and NetB (green, myc) are shown. Bottom panels: Higher magnification view of the boxed region in the top panel; the R8 and NetB::TM channels are displayed separately. Arrowheads bracketing the NetB::TM image mark the position of the linescan plotted to the right. Graph: Linescan of the fluorescence intensity in NetB::TM channel. Gray region marks background values. (c) Sample setup. Brain is shown in coronal section, viewed head-on. Body axes, (D)orsal-(V)entral and (R)ight-(L)eft, are marked. (d) Detail from (b) illustrates the imaged volume. (e) Three growth cones from the same WT brain. Panels were individually contrast enhanced to reveal dimmer features. See Figure 1—figure supplement 1 for a description of the image processing work-flow.
-
Figure 1—source data 1
Contains numerical data plotted in Figure 1b.
- https://doi.org/10.7554/eLife.20762.004
The findings in the R8 system are consistent with Net acting as a chemoattractant, a secreted ligand diffusing to form a gradient, which promotes extension of growth cones to the source of the ligand. However, a different interpretation was favored, one that proposed a local function for Net-DCC at M3 in target layer recognition (Timofeev et al., 2012). Central to this conclusion was the observation that a membrane-tethered variant of Net, expressed from the endogenous locus, can support wild-type targeting. The same molecular strategy had been used in the Drosophila embryonic midline to establish that diffusible Net is not required for the Fra-dependent guidance of commissural axons (Brankatschk and Dickson, 2006). This seminal work solidified the short-range paradigm of Net-DCC mediated axon guidance in which a soluble gradient of the ligand is not required for function.
Notably, at both the embryonic midline and visual system, tethered Net is expressed in a graded fashion comparable to the secreted form. At the midline, the boundaries of Net expression largely reflect the extended lateral morphology of cells expressing Net, the midline glia (Brankatschk and Dickson, 2006). Similarly, although Net is prominently expressed in L3 growth cones within the M3 layer, it is also visible as a gradient along the R8 targeting path between M0 and M3 (see Figure 1b). Thus, while these studies are consistent with a Net requirement that is limited to the extent of the source cells, it is not known whether the observed graded distributions are important for axon guidance to the target—the peak of the Net signal.
Given the penetrance and expressivity of the fra and Net phenotypes in R8 (see below) and the genetic tools available in Drosophila to explore mechanisms of axon guidance, we used live imaging to explore R8 targeting to M3 in more detail. Here we report, through detailed quantitative analysis of hundreds of mutant and wild type growth cones in intact developing animals that R8 growth cones in Net mutants or R8 growth cones lacking Fra target from M0 to M3 in a fashion indistinguishable from wild type. That is, Net does not act as a chemoattractant nor does Fra act as a chemoattractant receptor. In addition, we present evidence suggesting that R8 growth cones can recognize the target layer without Net-Fra. Instead, Netrin, DCC, and TRIM9, a signaling component directly downstream from DCC (Alexander et al., 2010; Hao et al., 2010; Morikawa et al., 2011; Song et al., 2011; Winkle et al., 2016, 2014), are essential for attachment of a single leading process extended from R8 growth cones to the target layer. We propose that R8 growth cones reach and recognize the target layer independent of Fra, adhere to the target layer in a Fra-dependent step, and this adhesion is consolidated by a TRIM9-dependent step. These findings favor the notion that in Drosophila Net mediates adhesion to neuronal processes or the extracellular matrix (ECM) at the target layer rather than promoting directed outgrowth to or recognition of the target layer.
Results
Live imaging reveals that R8 targeting occurs via discrete steps
To compare wild-type and mutant R8 targeting, we devised a live imaging protocol to follow growth cones in intact pupae as they extend from M0 to M3 (Figure 1c–e, Figure 1—figure supplement 1, Video 1). This system is similar to one developed by Hiesinger and colleagues to study the cellular mechanism of neural superposition, the choreographed re-distribution of R1-6 growth cones from ommaditial bundles to lamina cartridges (Langen et al., 2015). In the medulla neuropil, Hiesinger and colleagues used ex vivo live imaging to study R7 targeting, specifically characterizing the role of the Ca2+-dependent cell adhesion molecule, N-cadherin, in mediating adhesive interaction between growth cones and the developing neuropil (Özel et al., 2015).
Medulla Registration.
The developmental roll of the medulla is corrected in preparation for growth cone segmentation and tracking.
Through analysis of hundreds of wild type R8 growth cones, we characterize four distinct steps of targeting (Figure 2a, Video 2). The first, extension, begins with the polarization of growth cones in M0; a single thin process appears at the medial side of each growth cone (~38 hAPF). For many growth cones, this process probes into the medulla with multiple excursions and retractions, at rates reaching 1 μm/min (Figure 2c). Extension ends at 46.5 ± 1.5 hAPF with stabilization, as the tips of the R8 processes settle at 10.5 ± 1.5 μm from M0 (Figure 2a,b). In the third step, elongation, the tips of R8 projections continue to move away from M0 at the much slower rate of ~2.2 μm/hr (Figure 2a,b).
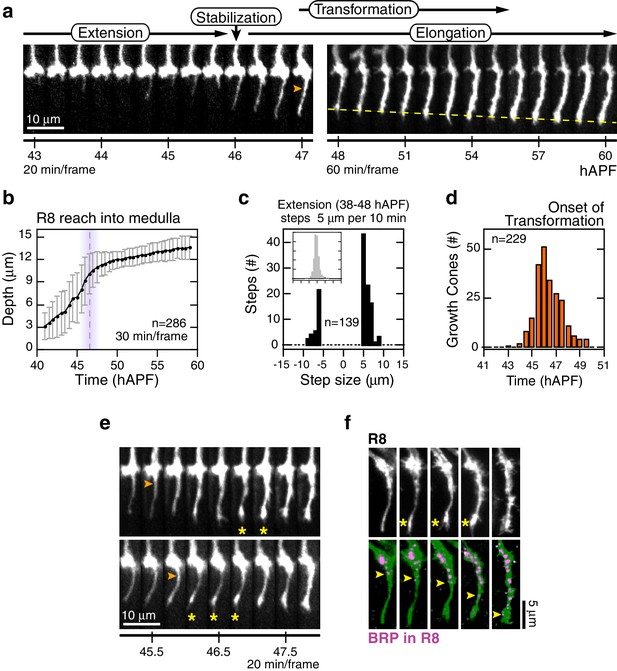
Wild-type R8 targeting.
(a) Steps of WT targeting. Orange arrowhead marks the onset of transformation. Dashed yellow line marks R8 depth through elongation. See also Figure 3—figure supplement 1b for an illustration of transient excursions from the target layer after stabilization. (b) Average reach of the R8 tip into the medulla (2 animals). Error bars are standard deviation. Dashed magenta line and band mark stabilization. (c) Counts of frame-to-frame (∆t = 10 min) tip movements equal to or greater than ±5 µm during extension (3 animals). Inset is the full distribution of steps, the tails of which are plotted in the parent graph. (d) Onset of transformation (3 animals). (e) Transformation proceeds from both ends. Orange arrowheads mark the first frames in which the proximal length of the thin process begins to expand. Yellow asterisks mark the expansion of the tip; see also (f). (f) Brp, a marker for presynaptic differentiation, accumulation follows anterograde expansion (yellow arrows) during transformation. Panels show confocal images taken at 47–49 hAPF. R8s are labeled with myr::GFP. R8s express V5-tagged Brp using the STaR system (Chen et al., 2014). Overlay of the Brp channel with a mask of the GFP channel highlights R8-localized puncta in magenta.
-
Figure 2—source data 1
Contains numerical data plotted in Figure 2b,c,d.
- https://doi.org/10.7554/eLife.20762.009
Aligned WT Growth Cones.R8 growth cones are aligned through the time series and extracted from the full image volumes. myr::tdTOM expressing WT growth cones from one brain are shown.
https://doi.org/10.7554/eLife.20762.010Elongation may represent the final step of active growth toward the target layer. Alternatively, R8s could be increasing their lengths in concert with the growing medulla. To distinguish between these possibilities, we compared the reach of R8 projections to a fiducial marker for an outer medulla layer, the Dm3 neurons (Figure 1a). In the adult, Dm3 processes project across columns to weave a meshwork at the M2/M3 layer boundary, a position that corresponds to 67 ± 5% of the depth of R8 projections (Figure 3a). Between 45 and 60 hAPF, the Dm3-defined layer is gradually displaced from M0 at an average rate of 0.9 μm/hr (Video 3). Since the projections of these neurons extend orthogonally to medulla columns, the measured movement captures the expansion of the neuropil. During elongation, the ratio of the distance between M0 and the Dm3 layer and the reach of individual R8 projections is 61 ± 4% (Figure 3d and Figure 3—figure supplement 1), suggesting that the R8s are increasing in length in concert with the growing neuropil. This result indicates that the target layer is reached at stabilization. It is possible that R8 axons actively and independently elongate to match the growth of the tissue. However, both elongation and medulla expansion continue through pre-synaptic differentiation (Chen et al., 2014 and below), arguing that the concerted growth is supported by attachments to neighboring cells and the target layer. We conclude that R8 projections recognize and become attached to their targets before elongation, at the stabilization step, and are stretched in length as the target layer moves away from M0.
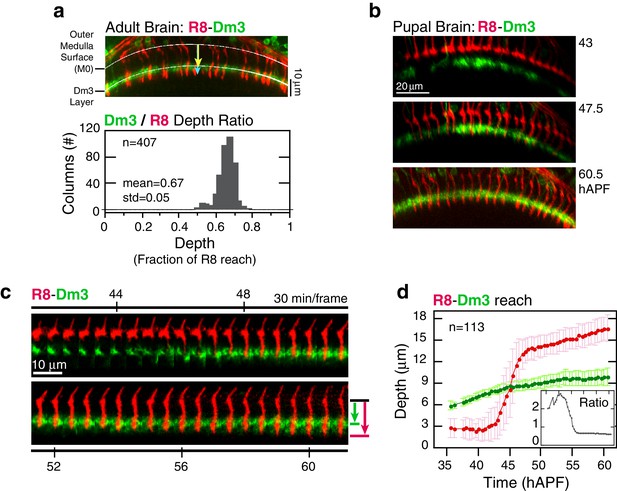
Analysis of elongation.
(a) Panel: Confocal micrograph of the outer medulla in the adult brain. R8s (red, myr::tdTOM) and Dm3s (green, myr::GFP) are shown. White arcs are fits to M0 and to the Dm3 processes. Arrows, yellow and blue, mark the distance from M0 to the Dm3 layer and to the R8 tips. Graph: Ratio of Dm3 and R8 depths in the brain from panel (n = 407). (b) Live imaging of R8 and Dm3. Panels from live imaging of the adult brain in (a). The view presented, matching the confocal micrograph in (a), was generated post-processing. Panels were individually contrast enhanced to reveal dimmer features. Note that Dm3 processes complete their expansion into layer M2-3 during the window of observation. Despite the incomplete coverage at early time-points, the representation of Dm3 throughout the time-series is sufficient to calculate a surface fit to this fiducial layer marker (see Materials and methods). (c) Time series of an R8 and underlying Dm3 process, a fiducial marker for the M2/M3 boundary. Red and green arrows illustrate the measurements plotted in (d). (d) Average reach of the R8 tip (red arrow in (c)) and the Dm3 distance from M0 (green arrow in (c)), measured in one brain. Error bars are standard deviation. Inset: Ratio of the Dm3 distance to M0 and the R8 tip reach. Mean ratio between 50–60 hAPF is 0.65 ± 0.06.
-
Figure 3—source data 1
Contains numerical data plotted in Figure 3a,d.
- https://doi.org/10.7554/eLife.20762.012
Two Channel Imaging of R8 and Dm3.
Live imaging of R8 (myr::tdTOM) and Dm3 (myr::GFP).
The first three steps of targeting are defined by the position and dynamics of the R8 tip; the fourth step, transformation, is a morphological change that begins after stabilization and overlaps with elongation (Figure 2a,d). At the onset of transformation, the original growth cone volume at M0 shrinks while the thin process fills out. The progress of transformation is not purely anterograde. In many cases, the apparent flow of material from the growth cone into the proximal length of the process and the expansion at the distal tip are distinguishable (Figure 2e). In the following ~5 hr, the R8 terminal takes on its mature form, which will eventually contain ~50 en passant synapses (Chen et al., 2014; Takemura et al., 2013). To ask how transformation relates to pre-synaptic differentiation, we studied the distribution of Brp in R8 projections using the STaR system (Chen et al., 2014), which enables cell-type specific tagging of proteins expressed at endogenous levels. We found that this marker for pre-synaptic active zones begins to populate the length of the R8 projection with the onset of transformation, following the anterograde expansion to eventually reach the expanded tip at the target layer (Figure 2f). Thus, while transformation is linked to pre-synaptic differentiation, the expansion of the R8 tip at the target layer is an earlier and distinct sub-step.
Re-visiting the Net-fra phenotypes
One potential challenge to examining the role of Net-Fra in R8 targeting is the discrepancy between franull(fra3 [Kolodziej et al., 1996], referred to as franull in the main text) and Net phenotypes reported by Salecker and colleagues. In genetically mosaic animals where fra is removed specifically from R8s, ~90% fail to reach the M3 layer (Timofeev et al., 2012). In contrast, the failure rate in whole animal Net mutants (NetA and NetB double mutant, referred to as Net null in the main text) (Newquist et al., 2013) is ~50% (Timofeev et al., 2012). These data raise the possibility of a second Fra ligand acting in parallel with Net. To clarify the interpretation of the live imaging data in the context of this ambiguity, we re-visited the mutant analysis through quantitative comparison of adult phenotypes.
In the adult brain, wild-type R8 projections reach 16.9 ± 1.7 μm into the medulla (Figure 4a, top graph). The spread in this distribution is partly due to the 3D organization of this tissue; the absolute distance between layers changes along the two principal axes of the neuropil. Wild-type R8 projections, when expressed as a fraction of the distance between M0 and M6, reveal a more narrowly defined (0.75 ± 0.05) target layer (Figure 4a, bottom graph). In addition to reducing variation within one brain, this normalization approach also enables more reliable quantitative comparisons to be made between R8s from different animals and different genetic backgrounds. For franull R8 terminals, the normalized depth is well-described as a mixture of three distributions (Figure 4b and see Materials and methods). All three components fall short of the target layer. Thus, removing Fra from R8s yields a phenotype with nearly full penetrance (i.e. the number of R8s affected) and variable expressivity (i.e. the severity of the R8 phenotype).
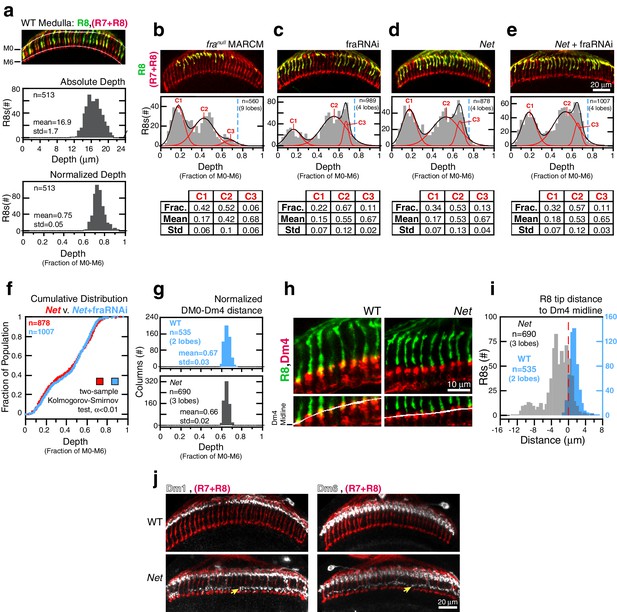
Fra and Net are in the same genetic pathway for R8 targeting.
(a) Panel: Confocal micrograph of outer medulla. R8s (green, ~70% expressing myr::GFP) and all R cells (red, labeled with Mab24B10) are shown. Graphs: Absolute (top) and normalized (bottom) distance between M0 and the R8 tips measured in medulla. (b) Panel: Confocal micrograph of outer medulla in a MARCM brain. franull R8s (green, expressing UtrnCH::GFP) and all R cells (red, labeled with Mab24B10) are shown. Projection depths of franull R8s were measured using a membrane-targeted marker (myr::tdTOM, not shown). Graph: Normalized franull R8s projection depths. Components (C1-3) for the Gaussian Mixture Model (GMM) fit to the distribution are plotted in red; black trace is the sum of the components. Dashed blue line marks the mean of the WT distribution from (a). Table: GMM parameters. Frac.: fractional contribution of each component to the fit. (c) Panel: Adult brain in which fra expression was knocked down in R8s using a cell-type specific driver (i.e. fraRNAi). Cell labeling as in (a). Graph: Normalized projection depths for fraRNAi R8s. Table: GMM parameters. (d) Panel: Net adult brain. Cell labeling as in (a). Graph: Normalized R8 projection depths in Net animals. Table: GMM parameters. (e) Panel: Net adult brain in which fra expression was knocked down in R8s using a cell-type specific driver (i.e. fra RNAi). Cell labeling as in (a). Graph: Normalized R8 projection depths in the Net+fra RNAi background. Table: GMM parameters. (f) Cumulative distribution of data in (d) and (e). The distributions are not distinguishable by the two-sample Kolmogorov-Smirnov test at significance level α < 0.01. (g) Comparison of the normalized distance from M0 to the midline of the Dm4 processes near M3 in WT (top, blue) and Net (bottom, gray) male adult brains. (h) Confocal micrographs of the outer medulla in WT (left) and Net (right) adult brains. R8s (green, myr::GFP) and Dm4s (red, myr::tdTOM) are shown. White curves are fits to the Dm4 midline. (i) Absolute distance between R8 tips and the Dm4 midline (dashed red line at 0) measured in WT (blue) and Net (gray) adult brains. Positive values indicate that the R8 tip is past the Dm4 midline. (j) Two additional cell types with altered morphologies in the Net background. Dm1 and Dm6 are both multi-columnar amacrine cells with processes at M1 in the WT (top row). Both cell types generate extra arborizations at M4-M5 (bottom row, yellow arrows) in Net animals. Dm1s and Dm6s expressing myr::tdTOM (white) were visualized with immunohistochemistry. Mab24B10 was used to stain for all photoreceptors (red).
-
Figure 4—source data 1
Contains numerical data plotted Figure 4a,b,c,d,e,f,g,i
- https://doi.org/10.7554/eLife.20762.017
Visual inspection of R8s in Net null animals suggests that a large fraction of R8s project to the M3 layer (Figure 4d, panel). However, the normalized depth for R8s in this genetic background can also be described as a mixture of three distributions, and the deepest reaching component (Figure 4d, graph and table) is nearly identical to that found in fra R8s (Figure 4b, graph and table) in terms of mean and standard deviation. In other words, the R8 depth distribution in the Net null background is well-described without a wild-type component (Figure 4a, bottom graph). To directly measure the distance between the R8 tips and the M3 layer in Net null animals, we used a Net-insensitive (Figure 4g) M3 marker, the Dm4 neuron (Figure 1a and Figure 4h). In wildtype, 95% of R8 tips reach past the Dm4 midline (Figure 4i). In contrast, only 19% of R8s in Net null animals extend this far. The majority of this 19% would belong to a sub-population distinct from wild type (i.e. tail of a mutant distribution); a much smaller fraction would be drawn from a purely wild-type component.
We conclude that the penetrance of the Net mutation for R8 targeting is much higher than originally reported and that the difference between franull and Net R8 targeting defects is one of expressivity.
To study the fra-Net genetic interaction, we performed epistasis analysis by reducing Fra levels in R8 cells with RNAi in the Net null background (Figure 4e). The phenotype of the double mutant was indistinguishable from Net null (Figure 4f). This result indicates that Fra and Net, as expected from a receptor-ligand pair, act in the same genetic pathway for R8 targeting. It also rules out the possibility of a second Fra ligand, acting in parallel with Net. However, the epistasis analysis does not rule out possible cell-autonomous contribution of other Net receptors. Indeed, the activity of such receptors in R8s would be consistent with the expressivity difference observed between the Net and franull genetic backgrounds.
We assessed this possibility for Dscam1 and UNC-5 with genetic mosaic analysis and did not find any obvious defects in R8 targeting. These results are consistent with the findings of a recent a cell-type specific RNA sequencing study (Tan et al., 2015), which showed that, at the onset of targeting, Fra is the only Net receptor expressed in R8 above 1–2 RPKM, the common threshold for noise in these types of studies.
Together, the epistasis analysis and the lack of evidence for the R8-specific activity of other Net receptors indicate that the difference in expressivity between the franull and Net phenotypes is due to a non-cell autonomous, pleiotropic effect of the Net mutation. Consistent with this finding, we found two other cell types with altered morphologies in the Net background (Figure 4j).
Why is the expressivity of the Net whole animal phenotype on R8 growth cones less severe than the removal of Fra selectively from R8s in mosaic animals? What is surprising is not that they are different, but rather that the pleiotropy is associated with weaker R8 expressivity. Removing Net from the whole animal alters the development of multiple neuronal classes in the medulla. Some, like R8, may be affected through the Fra pathway, while loss of Net signaling through the UNC-5 receptor may be important in other cases. We suspect that some aspect of the micro-environment in which R8 growth cones develop becomes more permissive to the progress of transformation in the absence of Net (see below). Regardless of the specific causes of pleitropy, the altered medulla environment in Net animals confounds mechanistic interpretation of R8 growth cone defects.
For a more refined approach to studying the role of Net in R8 targeting, we pursued two strategies involving genetic manipulation of the L3 lamina monopolar neuron, the principal source of Net in the target layer (Pecot et al., 2014; Timofeev et al., 2012). First, we ablated L3s through cell-type specific RNAi-mediated knock-down of a neurotrophic receptor. This experiment was originally published by our group where we showed that removal of all L3s led to a loss of Net from the M3 layer (Pecot et al., 2014). To compare the development of R8s with and without a home-column L3 in the same brain, we reduced the efficiency of the knock-down to achieve a partial ablation of the L3 array (~80% loss with ~20% surviving through eclosion). In this setup, all R8s with an L3 in the home column target as wild type. With R8s lacking a home column L3, we did observe targeting defects, but the phenotype was too variable to discern general patterns of growth cone behavior and draw mechanistic conclusions. We suspect that ablating L3, a major resident of the M3 layer, is too blunt a perturbation with pleiotropic consequences that go beyond removing a single secreted ligand.
As a second approach, we followed the targeting of wild-type R8s in columns with Net null L3s in genetic mosaics (i.e. MARCM) using live imaging. We did not observe any overt R8 phenotypes in this genetic background. The principal caveat here is that with MARCM in the lamina, mutant neurons appear as singlets or in small patches in an array of wild-type cells. We could not achieve mutant L3 patches large enough to ensure that any R8 sharing a column with a Net null L3 did not also neighbor at least one wildtype L3. Indeed, at 40 hAPF, L3 growth cones are very large along the dorso-ventral axis and extend into adjacent columns. Thus, it is likely that Net is contributed from wild-type L3 growth cones in neighboring columns.
In summary, the quantitative re-assessment of the mutant phenotypes and the epistasis analysis establishes that, for R8 targeting, removing either Net or Fra are equivalent perturbations. Thus, while it has not been possible to directly study the cell-type specific effect of removing Net on R8 targeting, we sidestepped the complications of Net genetics by focusing our efforts to characterize the role of Net-Fra signaling on comparing the dynamics of wild-type and franull-mutant growth cones in genetically mosaic animals.
Net-Fra signaling is not required for chemoattraction but promotes adhesion to the target layer
To study the role of Fra in R8 targeting, we combined live imaging with MARCM, a genetic strategy that relies on mitotic recombination to generate positively marked mutant cells in otherwise wild type tissue (Lee and Luo, 1999). In this experimental design, we also incorporated a direct labeling scheme, to mark all R8s independent of genotype. This enabled us to compare franull and wild-type R8 growth cones projecting into the same wild type medulla (Figure 5 and Video 4). Due to variations in the M0-to-M3 distance between different columns in the same wild type animal and between different samples, this internally controlled setup was essential for the detailed comparisons between wild type and mutant growth cones described below.
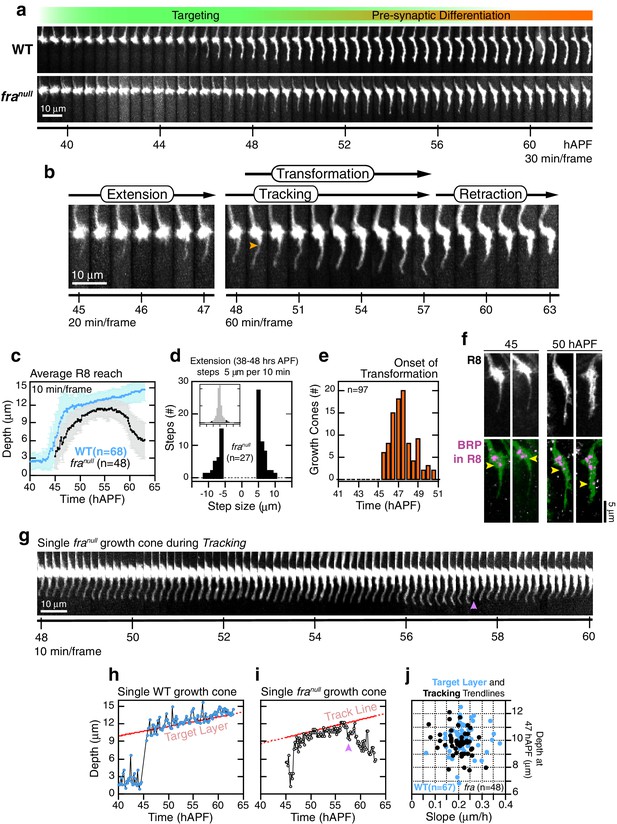
franull R8 targeting.
(a) Wild-type and franull growth cones from the same mosaic brain. (b) Steps of franull targeting. Orange arrowhead marks the onset of transformation. (c) Data for WT and franull R8s from the same brain presented as in Figure 2b. Note that the apparent difference between WT and franull reach prior to retraction is due to the opposing effects on this population average metric of (1) transient extensions beyond the target layer in the WT (Figure 3—figure supplement 1b) and (2) the retractions of mutant growth cones during tracking (see text). For other datasets in which the difference in average reach is less pronounced, see Figure 5—figure supplement 1c,d. (d) Data for franull R8s presented as in Figure 2c. (e) Data for franull R8s presented as in Figure 2d. (f) Brp accumulation follows anterograde expansion (yellow arrows) during transformation in franull growth cones. Panels show confocal images of franull R8 growth cones in MARCM brains at 45 and 50 hAPF. R8s are labeled with myr::GFP and myr::tdTOM (MARCM label, not shown). R8s express V5-tagged Brp using the STaR system (19). Overlay of the Brp channel with a mask of the GFP channel highlights R8-localized puncta in magenta. (g) franull dynamics during tracking; growth cone in (b) reproduced at full time resolution. Magenta arrowhead marks a transient retraction. See Figure 5—figure supplement 2 for more examples. (h) Tip trace of the WT growth cone in (a), plotted at 10 min resolution. The target layer, in red, is calculated as described in Materials and methods. (i) Tip trace of the franull growth cone in (b) and (g), plotted at 10 min resolution. Magenta arrowhead marks the transient retraction shown in (g). The track line, in red, is calculated as described in Materials and methods. (j) Scatter plot of slopes and positions of target layer and tracking trendlines at 47 hAPF, for the WT and franull growth cones in (c). The 47 hAPF position is a surrogate for the y-intercept of the trendlines.
-
Figure 5—source data 1
Contains numerical data plotted Figure 5c,d,e,h,i,j.
- https://doi.org/10.7554/eLife.20762.019
Two Channel Imaging of WT and fra R8s with MARCM.
Dual labeling of all (myr::tdTOM, red) and franull(UtrnCH::GFP, green) R8s reveals dynamics of wild-type and mutant growth cones in the same MARCM brain.
R8 growth cones reach the target layer without fra in a fashion indistinguishable from wild type. Extension is unaffected in mutant growth cones; a single thin process appears on the medial side and reaches into the medulla as in wild type (Figure 5a,b,d and Figure 5—figure supplement 1a,b). At ~48 hr, the tip of the thin process from these growth cones stalls at ~10 μm from M0 (Figure 5a,b), the approximate position of the target layer recognized by wild-type growth cones. At this point, franull development diverges from wild type in two respects. First, the distal tips of franull processes do not expand (Figure 5a,b,g and Figure 5—figure supplement 2). That is, while subtle dilations or other distinct structures may appear at this active site, these are invariably transient and are remodeled within 20–30 min to restore the thin morphology of the targeting process. Second, mutant processes do not stably adhere to the target layer. Instead, for the next ~10 hr (Figure 5—figure supplement 1e), as wild-type R8s passively elongate (Figure 5h), franull R8s actively follow a slowly advancing front in the medulla (Figure 5g,i and Figure 5—figure supplement 2), before they ultimately retract. That is, mutant R8s continue to extend and retract their thin processes, as their reach into the medulla increases over time. We term this behavior tracking.
We sought to assess whether franull growth cones track the moving target layer. For each wild type and franull growth cone in mosaic brains, we calculated the trendlines followed by the advancing R8 projections during elongation and tracking (see Materials and methods). Greater than 95% of franull R8s yielded tracking trendlines that are consistent with target layer trendlines of their wild-type counterparts (Figure 5j and Figure 5—figure supplement 1c,d). Thus, franull R8s track the target layer. This indicates that mutant R8 growth cones recognize one or more determinant of target layer specificity. The observation of tracking uncouples target layer recognition and attachment. In R8s, target layer recognition does not require the Net-Fra pathway.
The onset of transformation occurs on schedule in franull mutants (Figure 5e); however, in the absence of tip expansion, the progress of this morphological change is now only anterograde. As in wild type, Brp puncta enter mutant R8 projections following this anterograde expansion (Figure 5f). The progress of transformation is slow and fails to reach the target layer in the vast majority of franull growth cones before the final retraction after tracking (Video 5). That is, while synaptogenesis is affected in franull mutants, this is a downstream consequence of the earliest observable phenotypes—lack of tip expansion and stable attachment. The penetrance of the loss of target layer adhesion in franull growth cones is complete, consistent with our quantitative analysis of the adult phenotype. Live imaging of R8 targeting in Net null animals revealed similar behavior (Figure 6). In summary, the Fra-Net pathway is required for the attachment of the R8 terminal to M3 (Figure 8a). The marked expansion at the tip in wild type is consistent with Net-Fra signaling coupling a cytoskeletal response to substrate adhesion.
Aligned franullGrowth Cones
R8 growth cones are aligned through the time series and extracted from the full image volumes. myr::tdTOM expressing franullgrowth cones from one brain are shown.
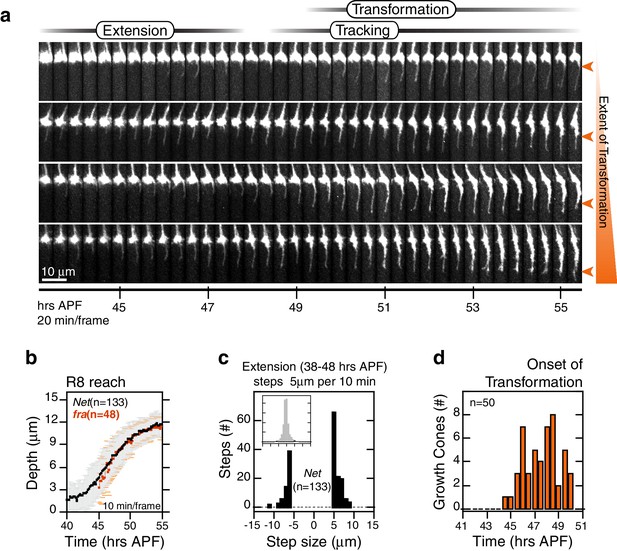
R8 targeting in Net mutants.
(a) Four growth cones from the same Net null mutant brain. Orange arrowheads mark the extent of transformation at the end of the time series in this data set; this determines the final R8 depth after retraction of the thin process. (b) Data for R8s in a Net mutant brain presented as in Figure 2b. The corresponding trace for franull R8s from Figure 5c is reproduced in orange. (c) Data presented as in Figure 2c. (d) Data for presented as in Figure 2d.
-
Figure 6—source data 1
Contains numerical data plotted in Figure 6b,c,d.
- https://doi.org/10.7554/eLife.20762.026
Trim9 is required to consolidate tip expansion
We sought to further test whether the Net-Fra pathway mediates adhesion. To do this, we assessed the function of another gene in the Net-DCC pathway that has cell-autonomous function. Trim9 has been identified as a component of Net-DCC signaling in neural development in both vertebrates and invertebrates (Alexander et al., 2010; Hao et al., 2010; Morikawa et al., 2011; Song et al., 2011; Winkle et al., 2016, 2014). Trim9 interacts through its SPRY domain with the Fra C-terminal cytoplasmic tail (Morikawa et al., 2011).
Trim9 is required in R8 targeting, as assessed in fixed preparations in the adult. The vast majority of Trim9null (Trim991 [Morikawa et al., 2011], referred to as Trim9null in the main text) R8s analyzed in genetically mosaic animals fall short of the target layer (Figure 7b). The small (~1%) fraction of mutant R8s that do overlap the wild-type depth distribution (Figure 7a) may represent a sub-population that targets as wild-type, or these may be the outliers of the mutant distribution. We conclude that, like franull, the Trim9 targeting phenotype exhibits near-complete penetrance. To explore the genetic interaction between Trim9 and Fra, we performed epistasis analysis by reducing Fra levels in R8 cells with RNAi in the Trim9 MARCM setup (Figure 7c,d). The phenotypes of double mutant and Trim9null single mutant R8s were indistinguishable (Figure 7e). Thus, Trim9 and Fra are in the same pathway for R8 targeting.
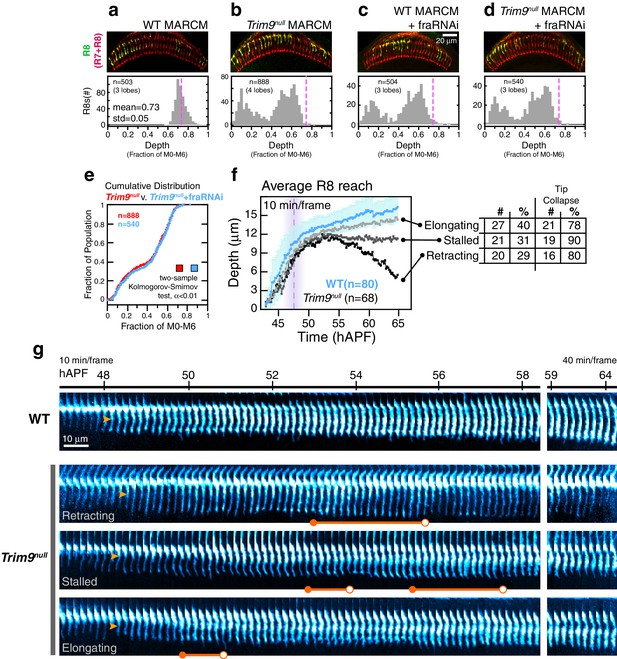
Trim9null R8 targeting.
(a) Panel: Confocal micrograph of outer medulla in a WT MARCM brain. MARCM labeled WT R8s (green, expressing UtrnCH::GFP) and all R cells (red, labeled with Mab24B10) are shown. Graph: Normalized R8s projection depths. Dashed pink line marks the mean of the distribution. (b) Panel: Confocal micrograph of outer medulla in a Trim9null MARCM brain. Cell labeling as in (a). Graph: Normalized R8s projection depths. Dashed pink line marks the mean of the WT distribution from (a). (c) Panel: Adult brain in which Fra expression was knocked down in R8s using a cell-type specific driver (i.e. fraRNAi) in the WT MARCM setup. Graph: Normalized R8s projection depths. Dashed pink line marks the mean of the WT distribution from (a). (d) Panel: Adult brain in which Fra expression was knocked down in R8s using a cell-type specific driver (i.e. fraRNAi) in the Trim9null MARCM setup. Graph: Normalized R8s projection depths. Dashed pink line marks the mean of the WT distribution from (a). (e) Cumulative distribution of data in (b) and (d). The distributions are not distinguishable by the two-sample Kolmogorov-Smirnov test at significance level α < 0.01. (f) Reach of the tip into the medulla for WT and Trim9null R8s, compiled from 2 brains. Error bars are standard deviation for WT. Dashed magenta line and band mark stabilization. Table shows number and percentage of mutant R8s in each class and for tip collapse events. As virtually all R8 terminals in the adult fall short of the target layer, the elongating class of R8 terminals must retract after 65 hr APF. (g) Wild-type and Trim9null growth cones from the same mosaic brain. Images are shown with a cyan-hot look-up table to increase displayed dynamic range. Orange arrowheads mark the onset of transformation. Orange barbells mark prominent expanded tips (closed end) that collapse (open end).
-
Figure 7—source data 1
Contains numerical data plotted Figure 7a,b,c,d,e,f.
- https://doi.org/10.7554/eLife.20762.028
We analyzed how Trim9null targeting defects arise using live imaging. Like fra, Trim9null R8s exhibit wild-type extension dynamics, their thin targeting processes reach depths comparable to their wild-type counterparts at the stabilization step (Figure 7f), and the onset of transformation occurs on schedule (Figure 7g, orange arrowheads). By contrast to franull, we observe tip expansion in ~90% of Trim9null R8s (Figure 7g, orange barbells, closed ends). The expanded tips of Trim9null R8s frequently collapse (82%; Figure 7g, orange barbells, open ends)—an event never seen in the wildtype after stabilization. Some of these tips exhibit multiple rounds of expansion and collapse. Tip collapse was also commonly observed in mutant R8s that had apparently completed transformation (Figure 7g, bottom panel). In franull R8s, tip expansion was never observed and this correlated with a lack of stable target layer adhesion. This, together with the observation that the tips of most Trim9null R8s expand but nearly all subsequently retract from the target layer (Figure 7b), suggest that Trim9 acts downstream of Fra to consolidate the attachment of R8 growth cones to the target layer.
During the time interval corresponding to elongation in wildtype, Trim9null R8s exhibit one of three types of behavior (Figure 7f). Retracting Trim9null R8s (30%) are distinguished from fra mutants during this period only by their unstable expanded tips (Figure 7g, second panel from top); this class tracks the target layer up to ~55 hAPF and retracts within the time of observation. Stalled Trim9null R8s (30%) are similar to the retracting class except that they maintain the depth they have achieved by ~55 hAPF (Figure 7g, third panel from top). The last class, elongating Trim9null R8s (40%), appear to complete transformation and elongate along with their wild type counterparts (Figure 7g, bottom panel). As virtually none of the mutant R8s are found at the appropriate target depth in the adult (Figure 7b) and tip collapse was observed in 78% of the terminals during development, this last class of Trim9null R8s must retract from the target layer sometime between 65 hAPF and eclosion (100 hAPF). We note that, similar to what is seen in the Net null background (Figure 6a), the efficiency of anterograde progression of transformation correlates with the three classes—that is, retracting Trim9null R8s show minimal transformation while in all elongating Trim9null R8s the process appears complete. We do not know the factors that influence transformation efficiency. While the onset of transformation is independent of Fra, Net, and Trim9, it is possible that the anterograde progression of transformation is linked to events regulated by these genes, such as tip expansion and target layer adhesion. We suspect, given that transformation represents pre-synaptic differentiation, that the extracellular contacts made during this process prevent complete retraction of the mutant R8 processes back to M0, producing the expressivity spectrum observed in all three genes examined.
The analysis of Trim9null supports the conclusion that the Net-Fra pathway regulates adhesion of R8 growth cones to their targets (Figure 8a).
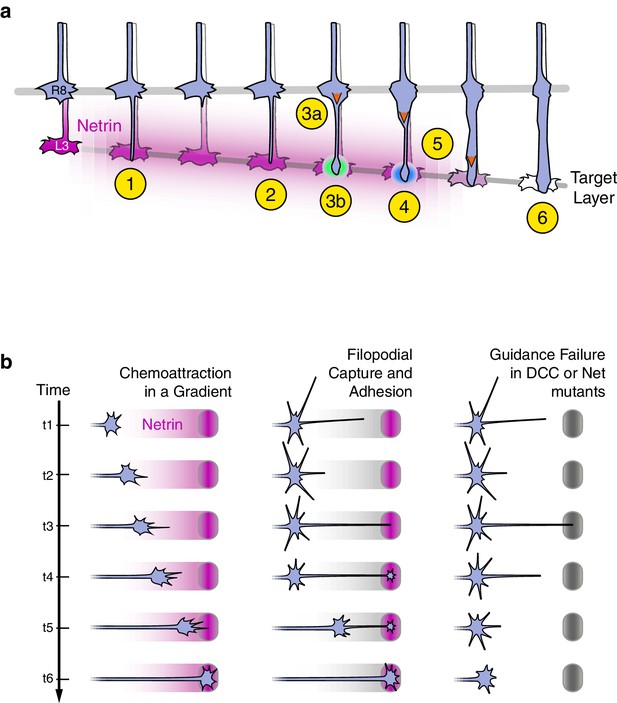
Axon guidance through Net-DCC-mediated adhesion.
(a) Net-Fra signaling in the second step of R8 targeting: (1) Extension of thin process into medulla, Net-Fra independent. (2) Target layer recognition, Net-Fra independent. (3a) Onset of transformation, proximal expansion (orange arrowhead), Net-Fra independent. (3b) Tip expansion in the target layer, Net-Fra dependent (cyan highlight). (4) Consolidation of adhesion to the target layer, Net-Fra-Trim9 dependent (blue highlight). (5) Progress of transformation (orange arrows). The extent of transformation completed before the end of tracking in the mutant backgrounds may underlie the difference in expressivity between the Net, franull, and Trim9null adult phenotypes. (6) Elongation and maturation. (b) Net-DCC mediated guidance works through target adhesion, not chemoattraction in a gradient. First Column: In the classical view of chemoattraction, DCC-laden filopodia sense the gradient of extracellular Net (pink) and direct the movement of the growth cone toward the source. Second Column: In the alternate model, the Net source is detected independent of the gradient (gray), through filopodial search and capture (t1-t3) or directed extension of a targeting filopodia guided by a separate mechanism (not depicted). Net-DCC signaling at the ligand peak promotes attachment to the target (t4); the growth cone proper then reaches the target with the aid of this initial anchor (t5-t6). Third Column: The generalized view of axon guidance failure in DCC-Net mutants shows that reaching the target does not require the receptor or the ligand; the final phenotype is due to retraction instead of a loss of attraction.
Discussion
Net-DCC is the prototypical ligand-receptor pair required for neural circuit assembly with known roles in diverse processes such as chemoattraction (Serafini et al., 1996), target recognition (Timofeev et al., 2012), and synaptogenesis (Colón-Ramos et al., 2007). Beyond the nervous system, Net signaling has been implicated in cell polarization (Ziel et al., 2009) and adhesion (Srinivasan et al., 2003; Yebra et al., 2003). The details of how Net-DCC affects development will, of course, be context dependent. While this complicates generalization, the diversity of developmental contexts provides different windows into studying this evolutionarily conserved signaling module. R8 is a superb example of this.
We sought to leverage the relative simplicity of R8 geometry and development, and the large numbers offered by the visual system to gain insight into how Net-DCC functions in neural circuit assembly through live imaging. Loss of function mutations in the genes for the ligand, the receptor, and a protein associated with the receptor result in strong end-point phenotypes that are consistent with lack of chemoattraction in a gradient, absence of target recognition, or failed synaptogenesis.
Direct visualization of live mutant and wild-type growth cones in genetically mosaic animals allowed us to distinguish between these possibilities. We argue that in R8 neurons, the Net-Fra pathway promotes attachment, not chemoattraction or target recognition. The onset of synaptogenesis is also Net-Fra independent, and while standard genetic analysis cannot rule out a subsequent role for Net-Fra in this process, the attachment function precedes synaptogenesis. Each R8 growth cone in all three mutant backgrounds studied—Net, fra, and Trim9—extends a single process which reaches the M3 layer as in wild type. The targeting defects observed in the adult arise from retraction from M3 as a consequence of the lack of the stable association and morphological transformation of the tip of the R8 growth cone at the peak of the Net gradient. We note that given the cellular and molecular complexity of the medulla neuropil, retraction may also reflect the presence of repulsive factors in M3 uncovered through loss of Net-based attachment. This, however, is not consistent with the tracking behavior of mutant R8s; the persistent association of the tip of R8 growth cones with the target layer (Figure 5—figure supplement 2) is difficult to reconcile with a repulsive cue at M3. Despite this and other possible contributions to the terminal phenotype, the simplest interpretation of our data is that the Net-DCC interaction promotes adhesion. For the remainder of the discussion, we use this term to describe the role of Net-DCC signaling in R8 targeting.
Importance of live imaging to explore mechanisms of axon guidance
Live imaging coupled with the unique features of R8 targeting and comparative analysis of wild-type and mutant growth cones in mosaic animals were essential to pinpoint the requirement for the Net-Fra ligand receptor pair for target adhesion. Specifically, the identification and analysis of the tracking behavior of mutant R8s would not have been possible in fixed preparations. Standard fixation techniques do not preserve tracking filopodia at their full lengths; the shortcomings of fixation have been highlighted previously for cytonemes (Kornberg and Roy, 2014) in the context of the response of cells to morphogens. In addition, the continuous, extended observation of individual growth cones was critical to recognizing general trends over transient dynamics. For example, even with perfect preservation, only half of the tracking R8 processes in fra mutants would appear to be at the target layer at any given time (Figure 5—figure supplement 1e). The simplest interpretation of such data would support the erroneous conclusion that half the R8 population required Net-Fra for chemoattraction. Thus, live imaging allowed us to discriminate between chemoattraction, target recognition, and retraction after reaching the target.
Net-DCC mediated adhesion in neuronal development
The canonical role of Net-DCC in neuronal development is axon guidance through chemoattraction: a gradient of soluble Net steers DCC-expressing growth cones to their targets. Adhesion is also a recognized output of Net signaling, particularly in non-neuronal systems (Srinivasan et al., 2003; Yebra et al., 2003). Biochemical and cell biological studies using dissociated vertebrate neurons or neural explants suggest that these different functions may share a fundamental mechanistic similarity. Structurally, Net can be described in three domains. The two N-terminal domains interact with cognate receptors, including DCC (Keino-Masu et al., 1996). The C-terminal domain of Net is highly charged and mediates non-specific adsorption to cell surfaces, ECM components, and to tissue culture substrates (Kappler et al., 2000; Moore et al., 2012; Yebra et al., 2003). In vitro, substrate-bound Net promotes adsorption of DCC-expressing cells (Shekarabi et al., 2005) and the Net-DCC interaction can withstand measurable pulling forces (Moore et al., 2009). Net’s ‘stickiness’ can be reduced by masking the charge with heparin or removing the C-terminal domain altogether (Moore et al., 2012). These manipulations do not alter the ligand’s interaction with DCC, but significantly reduce the neuronal response to Net in classic in vitro assays of Net-DCC function, including growth cone expansion, axon outgrowth, and turning (Moore et al., 2012). For example, in the explant turning assay, a cluster of Net-secreting cells abutting a dissected embryonic spinal cord can attract extending commissural axons over a distance of some 200 μm (Kennedy et al., 1994). Without the C-terminal domain, the range over which Net can attract axons is markedly reduced (Moore et al., 2012). Consistent with the importance of substrate binding to the function of externally supplied Net, most of the endogenous Net in the developing mouse spinal cord is cell- or ECM-bound (Kennedy et al., 2006). Together, these observations support the notion that “growth cones pull directly on the cues that guide them” (Moore et al., 2012), thereby unifying the chemoattraction and adhesion roles of Net-DCC in the concept of haptotaxis, or motility through traction.
The adhesion function of Net-DCC in vivo is well characterized in the anchor cell (AC), a non-neuronal cell in C. elegans. During development, the AC polarizes toward and invades the basement membrane, initiating the attachment of the uterus to the vulva. UNC-6 – UNC-40 signaling is required for the efficient completion of this process. In a series of studies using live imaging, Sherwood and colleagues demonstrated that UNC-6 localized to the basement membrane stabilizes UNC-40 clustering in the AC, which, in turn, directs polarized F-actin production to the basal surface of this cell (Hagedorn et al., 2013; Wang et al., 2014; Ziel et al., 2009). Without UNC-6, UNC-40 can still form clusters and promote actin assembly, but these patches of activity are transient and randomly positioned around the cell periphery (Wang et al., 2014). MADD-2, the C. elegans homolog of Trim9, is also required in the AC to maintain stable and appropriately polarized F-actin patches; without MADD-2, basal patches can be transient, mis-localized actin assembly is observed, and, ultimately, basement membrane invasion is compromised (Wang et al., 2014). The parallels between the use of Net-DCC-Trim9 in development by R8 and the non-neuronal AC—to effect a spatially constrained adhesion or polarization response at an acute presentation of Net (i.e. gradient peak for R8 and basement membrane enrichment for the AC)—raises the possibility that adhesion may be a widely conserved cell biological output of this signaling module.
In this context, R8 targeting and midline crossing in Drosophila may function in a fundamentally similar way through an adhesion-based mechanism. Brankatschk and Dickson demonstrated that the growth cones of commissural neurons, like those of R8, respond to a membrane-tethered form of Net in a fashion indistinguishable from the secreted form of Net (2006). If commissural axons can, indeed, reach the midline without this signaling module—that is, if the mutant phenotypes, as in the R8 system, arise due to retraction rather than a lack of attraction, the role of Net-Fra would be better described as enabling the axons to traverse the dense midline neuropil. Alternatively, commissural growth cones may extend towards the midline via haptotaxis, growth via traction (and hence adhesion) along a pathway of increasing levels of Net bound to the surface of midline glia or associated ECM. Thus, given our current state of knowledge of midline crossing, it is possible that Net-Fra act through an adhesive mechanism in this system.
Evidence consistent with this view comes from the work of Emoto and colleagues, who carried out a detailed analysis of Trim9 function in the C4da neurons of Drosophila (Morikawa et al., 2011). The branched axon terminals of these sensory neurons send projections that cross the midline of the ventral nerve cord (VNC), the larval counterpart of the embryonic midline. In Net, fra, and Trim9 mutants, these projections are severely reduced or missing. There are three different sub-classes of C4da neurons and each subclass elaborates a characteristic number of contralateral projections. Neurons with more contralateral projections express higher levels of Trim9. Furthermore, overexpression of Trim9 produces ectopic projections in a dose-dependent manner. This overexpression phenotype is completely suppressed in a franull background (Morikawa et al., 2011). Given what we know of Trim9 function in stabilizing adhesion downstream of Net-Fra in R8s and polarization in the AC, the C4da results are consistent with Trim9 controlling the fine-tuning of midline crossing probability. The Net-Fra initiated midline contacts would then be either stabilized or lost depending on Trim9 levels, resulting in successful or retracted contralateral projections, respectively.
These observations in Drosophila as well as in vitro experiments with vertebrate growth cones raise the interesting possibility that Net-DCC-mediated adhesion may be a common mechanism used by growth cones in developing invertebrate and vertebrate brains.
Circuit assembly through contact dependent processes
Tessier-Lavigne and Goodman (1996) divided guidance signals into four categories. Short-range signals would act through direct contact between growth cones and cells or growth cones and the ECM to attract or repel growth cones. Conversely, long-range signals, secreted by cells, were proposed to act at a distance in a graded fashion to attract or repel growth cones.
In Drosophila, both at the midline and in the visual system, a membrane-tethered form of Net is sufficient to rescue guidance phenotypes (Brankatschk and Dickson, 2006; Timofeev et al., 2012). These results have been interpreted as evidence for a local or short-range function for Net-DCC. This is by contrast to the diffusible gradient view supported by the original conception of long-range chemoattraction and the results of turning assays of vertebrate growth cones in vitro. However, both at the midline and in the medulla, tethered Net is present as a gradient that peaks at the targets of guidance, and in the absence of studies testing the relevance of this cell-bound gradient to guidance, the distinction between the short- and long- range functions of Net-DCC remained unclear. Net is a secreted molecule of the laminin superfamily and, in principle, can diffuse to form a gradient. As noted above, however, data from in vitro studies argue in favor of the relevance of immobilized Net over that of a soluble form. Together, these results raise the possibility that Net-DCC guidance relies principally on immobilized Net, thereby eroding the mechanistic distinction between the short- and long-range guidance in a gradient.
In the R8 system, the gradient is not required. Our results show that the R8 growth cone can reach and recognize its target without the ligand gradient, or the receptor, in a fashion indistinguishable from wild type, and that Net-DCC act to promote adhesion at the source of Netrin. Despite the presence of Net along the path to M3, we cannot detect an effect of the ligand-receptor interaction while the tip of the R8 growth cone is moving up the Net gradient (Figure 5—figure supplement 1b). Such spatial specificity in signaling output may be achieved through fine-tuning the response of the system to the ligand concentration, or by inhibiting the activity of the receptor until the target is reached.
How R8 uses Net-DCC may be idiosyncratic to this neuron. That is, the evidence against gradient-based chemoattraction in R8 targeting does not rule out a role for this mechanism in other in vivo contexts. If Net does act in a graded fashion in other systems, we suggest, in agreement with the work from the Sheetz lab, that it does so not as a soluble factor, but rather as a substrate-bound molecule that influences growth cone behavior through contact. The distribution of the ligand may be determined by localized expression at a single source followed by diffusion and adsorption. Alternatively, a track of cells may form a gradient by expressing and presenting different levels of Net on their surfaces. Thus at a mechanistic level, we propose that, in vivo, DCC responds to immobilized, not soluble, Net. This initial adhesive interaction may stabilize attachment as in the case of R8 or, alternatively, this may promote traction for growth cone motility along a surface (i.e. haptotaxis).
This and other studies of Net-DCC signaling in invertebrates and vertebrates raise the possibility that Net and other secreted signals within the developing CNS may act principally in close proximity to the source cells, either associated with the surface of cells or the ECM, to elicit discrete and localized responses. In the absence of action at a distance, neural circuit assembly would proceed in a stepwise fashion where neuronal processes sample a complex environment through direct contact (i.e. via ‘touch’ rather than ‘smell’), integrate these signals and transform them into morphological and biochemical specification.
Materials and methods
Histology and confocal microscopy
Request a detailed protocolHistology was performed as described previously (Chen et al., 2014) with minor modifications. After antibody incubations, brains were washed into PBS with 0.5% Triton X-100 (PBT). To minimize tissue shrinkage, the brains were moved from PBT to mounting medium (EverBrite, Biotium) through a series of mixtures with increasing concentrations of the latter. The following primary antibodies were used: chicken Pab α-GFP (abcam, ab13970, RRID:AB_300798, 1:1,000), Mab24B10 (Van Vactor et al., 1988) (DSHB, RRID:AB_528161, 1:20), rabbit Pab α-DsRed (Clontech, Cat# 632496, RRID:AB_10013483, 1:200), mouse α-V5 (Serotec, Cat# MCA1360, RRID:AB_322378 1:200). The following secondary antibodies were used: Alexa Fluor 488 goat α-chicken, Alexa Fluor 568 goat α-rabbit, Alexa Fluor 647 goat α-mouse (ThermoFisher, Cat# A-11039, RRID:AB_2534096, Cat# A11036, RRID:AB_10563566, Cat# A-21235, RRID:AB_2535804, 1:500). Confocal images were acquired with a Zeiss LSM780 system.
Confocal image analysis
Request a detailed protocolTo carry out 3D measurements in the medulla, imaged volumes are deconstructed into oriented medulla columns bounded by computed surfaces for M0 and M6. A typical multi-channel confocal stack of the medulla measures 150 x 150 x 180 µm and has voxel dimensions of 0.29 x 0.29 x 0.4 µm. In a pre-processing step, all channels are scaled in the z-dimension to achieve unit voxel aspect ratio. The Mab24B10 channel (i.e. all R cells) is used in the deconstruction. R7 axon terminals are the deepest-reaching visible features; a mask of the R7 axon tips is generated by manually cleaning up the image. Local intensity maxima in the original image are selected with this mask and the resulting point cloud is used to define the continuous 3D surface of the M6 layer. The bundles of R cell axons that stretch across the outer medulla surface increases the complexity of the image at M0; a more involved approach is required to define this layer. The area bounding the footprint of the R7 projections in M6 is divided into 50–72 regions, and, for each region, columnar volumes orthogonal to the M6 surface are extracted from the image. Intensity profiles along the column axis of these volumes are used to find local peaks, which are classified according to magnitude and their distance from the M6 surface. These two parameters are used to identify the peaks that reside in the M0 layer; the M0 surface is built from a point cloud derived from the selected peaks. A similar approach is used to compute the Dm4 surface. To define the medulla columns, a sequence of masks laminating the space between the M0 and M6 surfaces are used to generate maximum intensity projections (MIPs) of the Mab24B10 image. The cross-sections of R7-R8 projections are identified as local intensity maxima in these MIPs. Across the MIP sequence, the intensity maxima are grouped into individual tracks that define the position and orientation of the medulla columns. This information is used to create single panel MIPs of individual R8 axons from the R8 channel. The tips of R8 projections are marked manually on the MIPs and the input is used to calculate 3D distances to the M0 and M6 surfaces. Up to 500 R8s per medulla were scored with this approach. This analysis was written in Matlab (Mathworks) with a critical script sourced form the Mathworks File Exchange repository (D’Errico, 2005). Fiji (ImageJ) (Schindelin et al., 2012) was used for user-assisted tasks.
Gaussian mixture modeling of adult R8 phenotypes
Request a detailed protocolEach data set in Figure 4b–e was modeled as mixtures of 2–7 Gaussian distributions using a built-in Matlab (Mathworks) function. For all cases, the Akaike information criterion, a fitting evaluation metric that weighs the goodness-of-fit against the number of free parameters, decreased monotonically until the minimum was reached at 4 or 5 components. While more complex models are supported by the data, we presented the fits with 3 components, the minimum number required to represent the major sub-populations. When modeled with >3 Gaussians, only the fraRNAi (Figure 4c) data supports a wild-type component with a mean at 0.75.
2P microscopy and image processing
Overview
Using a custom-built two photon microscope, we can image the developing visual system between 15 and 65 hr after puparium formation (hAPF). In the specific case of the R8 photoreceptor cell, one imaging session can capture the dynamics of 50–200 individual growth cones at 5–30 min time resolution. We analyzed the 3D time series using a suite of custom scripts implemented in Matlab (Mathworks). The source code for this suite of scripts are available as a supplement to this article.
Microscope
Request a detailed protocolThe microscope was designed to maximize light collection efficiency. The three principal considerations were: (1) High efficiency GaAsP detectors (Hamamatsu); (2) A short, wide-angle collection path with 2” optical elements; and 3. Use of a large field-of-view objective (Zeiss, W Plan-Apochromat 20x/1.0 DIC) that balances a long working distance with a large numerical aperture. A tunable Ti:sapphire pulsed laser (Chameleon Ultra II, Coherent) was used as the light source. Most of the images presented in this study were collected at ~25 mW (920 or 970 nm) under-the-objective power to minimize photobleaching. The microscope hardware and image capture was computer controlled and driven by ScanImage (Pologruto et al., 2003).
Sample preparation
Request a detailed protocolDuring pupal development, a small, fat-free window over each retina provides optical access into the visual system. The cuticle around the head is removed after head eversion (~12 hAPF), and the animal is attached eye-down on to a coverglass (22 x 50 mm, No.1.5) coated with dilute embryo glue. Up to 18 animals can fit on a sample slide making it possible to image multiple flies in a single session. To provide sufficient immersion liquid for the long imaging sessions, a water reservoir is constructed on the opposite surface of the cover glass. Glass-bound pupae are suspended above a second water reservoir to minimize dehydration. Animals are staged at white pre-pupa formation (0 hAPF) or head eversion (12 hAPF) and kept at 25°C using an objective heater system (Bioptechs).
In all live imaging experiments, genotype-independent R8 labeling was achieved by activating a transcriptionally silenced strong driver with a cell-type specific recombinase (Flp or R, driven by a promoter from the senseless gene, see Experimental Genotypes). The two drivers used, GMR and brp-2A-LexA (see Experimental Genotypes), offered different advantages and caveats. GMR-driven expression of myr::tdTOM provides strong labeling of R8s up to ~50 hAPF. The signal begins to degrade beyond this point due to early onset of pigmentation in the retina and the imaging window closes ~58 hAPF. The modified brp BAC, utilized as a LexA driver, complements the GMR promoter. Expression from brp-2A-LexA dips at ~45 hAPF, but recovers to reveal the details of R8 dynamics past 60 hAPF without significant loss of signal strength. While either strategy yields an adequate description of WT R8 targeting, combining the strengths of both was essential to a quantitative study of the franull phenotype.
Image processing and analysis
Request a detailed protocolA typical 10 min per frame 24 hr time series contains ~140 512 x 512 x 390 pixel stacks with voxel dimensions of 0.24 x 0.24 x 0.4 µm. In a pre-processing step, these stacks are scaled in the z-dimension to achieve unit voxel aspect ratio. Medulla registration begins with manual clean-up of a single time point, the anchor stack (40 hAPF), to remove contributions from the lamina and incoming R8 axons. The cleaned stack is used as a mask to select local intensity maxima in R8 growth cones. The resulting point cloud, which is a sparse representation of the outer medulla surface, is fit to an oblate ellipsoid, yielding the rotation matrix that brings the medulla into alignment with the image axes (i.e. top-down view.) The point cloud itself is aligned to the image axes and used to define the continuous 3D surface of the outer medulla. A mask that contains the R8 growth cones, the shell mask, is built as a slab centered around this surface. Through an iterative cross-correlation search in the rest of the time series, coordinates that most closely match a region-of-interest (ROI) near the center of the R8 array in the anchor stack are identified. An ellipsoidal mask is used to extract volumes-of-interest (VOIs) centered on these coordinates in each stack. Starting from the anchor stack and moving to either end of the time series, the VOIs are iteratively aligned to one another using rigid body transformations. The product of these transformations and the original rotation matrix bring the full time series into register with the anchor stack and align the medulla with the image axes.
Growth cone segmentation is performed in a single stack from the registered series, the seed stack (45 hAPF). The seed stack is processed to reduce noise and local intensity variations, masked with the shell mask, and flattened as a MIP. A 2D growth cone template is generated and manually edited to refine the segmentation. The resulting segmented growth cone mask is used to define the 3D center of each growth cone in the seed stack. A refined surface for the outer medulla is computed using the growth cone centers and medulla column vectors for the growth cones are calculated as normals to the new surface.
Growth cone tracking is carried out in a MIP representation of the time series, generated from medulla-registered stacks masked with the shell mask. The top-down view of the registered orientation minimizes overlap between R8 growth cones while the shell mask removes signal contribution from non-growth cone objects in the full image volume. Small 2D ROIs centered around each segmented growth cone in the seed stack are used to initiate a cross-correlation-based iterative search to find best matching regions in successive frames of the time series. XY tracks from this search are combined with Z coordinate information retained from the MIP generation step to compile the first-pass XYZT coordinates of the growth cone centers in the registered orientation.
In growth cone alignment, the 4D positions of R8 growth cones are refined in the original orientation of the raw data. Starting with the seed stack, a template VOI for each growth cone is extracted from the 3D position and masked with a cylinder oriented along the medulla column vector calculated in the segmentation step. Masked target VOIs are extracted from the next stack in the series, using 3D coordinates from the tracking step and vectors corrected with the medulla transformation matrix. Target VOIs are aligned to template VOIs with rigid body transformations and the aligned target VOIs are re-cast as the templates for the next iteration of the operation (i.e. next stack). This process has two principal outputs: (1) Refined 4D positions and orientations of R8 growth cones, and (2) Masked MIP series of individually aligned growth cones (see Figure 1d). To avoid reducing the spatial resolution of the MIP series, rotations about either axis of the imaging plane (XY) are suppressed during MIP generation. As a result, images shown under-represent the true 3D length of the R8 projections (see legend for Video 2).
R8 tip position relative to the growth cone center is tracked automatically through each aligned series; this output is visually inspected and corrected, when necessary. The accuracy of automatic tracking increases with image quality and peaks at ~95%. Onset of transformation times were scored manually.
Most image processing was done using custom software written in Matlab (Mathworks). Several critical scripts were sourced from the Mathworks File Exchange repository (D’Errico, 2005, 2009, Kroon, 2008). Fiji (ImageJ) was used for batch stack processing and user-assisted tasks.
Identifying target layer and tracking trendlines
Request a detailed protocolFor each growth cone tip trace, a family of candidate trendlines was generated using data points within a moving window of 10–12 hr. For WT growth cones, a subset of the data in each window was selected by fitting a lower-bound cubic spline to the data. The complexity of the spline (i.e. number of cubic functions used) was increased until 30% of the data points were within 0.5 µm of the curve. These spline-proximal points were used to define a line using the Thiel-Sen estimator method. The intercept of the line was adjusted so as to give 90% of all the data in the time window positive residuals. For franull growth cones, an upper-bound spline was fit to the data in each time window and, again, the complexity of the spline was increased until 30% of the data points were within 0.5 µm of the curve. The best-fit line to the spline-proximal points was refined using a 30% subset with the smallest standard deviation in their residuals to the line. For both classes of growth cones, the optimum trendline from among the candidates was selected using the product of three weight functions. The first two of these are normal distributions, which rank the slope and intercept of the trendlines based on parameters derived from the average tip trace curves of WT growth cones (e.g. blue mean and errors in Figure 5c). The third is an exponential decay function that ranks the standard deviation of the residuals to each candidate trendline. This analysis was written in Matlab (Mathworks) with a critical script sourced form the Mathworks File Exchange repository (D’Errico, 2009).
Experimental genotypes
Flies were reared at 25°C on standard cornmeal/molasses medium. Pupal development was staged relative to white pre-pupa formation (0 hAPF) or head eversion (12 hAPF).
Main figures
Request a detailed protocol1b, NetAΔ,NetB::TM/+;; senseless-R::pest, GMR-RpdOUT-myr::tdTOM/+
Description: One of the maternal X chromosomes carries a myc-tagged membrane-tethered variant of NetB (NetB::TM) expressed form the native genomic locus of NetB, in a NetA deletion background (Brankatschk and Dickson, 2006). Expression of GMR (Hay et al., 1994) driven myr::tdTOM is controlled by sens-R::pest, resulting in genotype-independent labeling of ~70% of R8s and ~3% of R7s.
1e, W; Sp-Cyo/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/+
Description: The R recombinase (Nern et al., 2011), under the control of the sens F2 fragment (Pepple et al., 2008), removes the RpdOUT transcriptional and translational interruption cassette from the modified brp BAC (STaR system, RRID:BDSC_55760) (Chen et al., 2014). The 3’ read-through and translation of the sequence downstream of the excised cassette leads to addition of the V5 epitope tag to Brp (not utilized in this experiment) and production of the LexA transcriptional activator as a discrete polypeptide. The expression domain of the sens F2 fragment is specific to R8s in the medulla; LexA driven myr::GFP labels ~70% of R8s and ~3% of R7s.
2a-d, same as 1e
2e, sens-FLP1/+; GMR-FRT-Stop-FRT-GAL4/+; brp-FlpdOUT-GFP-2A-LexA, UAS- FRT-Stop-FRT-myr::tdTOM/+
Description: FLP1 recombinase driven by the sens promoter fragment (RRID:BDSC_55768) excises the FRT-flanked transcriptional stop cassettes in the GAL4 and UAS elements, leading to myr::tdTOM expression in R8s. The stop cassette in the UAS element is not necessary for cell-specific labeling. The STaR element is included to visualize Brp puncta in the live preparation (not shown).
2f, Same as 1e
3, w; LexAop-myr::tdTOM, R25F07-LexAp65/+; senseless-R::pest, GMR-RpdOUT-myr::tdTOM/+
Description: R25F07-LexAp65 (RRID:BDSC_52703) drives expression of myr::tdTOM in Dm3 cells through pupal development into adulthood. R8-specific expression of myr::tdTOM achieved as in 1b.
4a, w; Sp-Cyo/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/+
Description: see 1e.
4b, ey3.5-FLP1/+; FRT42B, ACT-GAL80/FRT42B, fra3; sens-GAL4, UAS-UtrnCH::GFP/ sens-R::pest, GMR-RpdOUT-myr::tdTOM
Description: Variant of the MARCM genotype in 5a. The filamentous actin marker UtrnCH::GFP (Burkel et al., 2007) (provided by Margot E. Quinlan) is driven by sens-GAL4 and positively labels fra3 (Kolodziej et al., 1996) R8s. Expression of GMR (Hay et al., 1994) driven myr::tdTOM is controlled by sens-R::pest, resulting in genotype-independent labeling of ~70% of R8s and ~3% of R7s.
4c, w/Y; sens-GAL4/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/UAS-fra RNAi (DRSC HMS01147)
Description: The three elements on the third chromosome use the STaR system to label ~70% of R8s with myr::GFP (see genotype description for 1e). sens-GAL4 drives expression of the short hairpin RNAi construct directed at fra (RRID:BDSC_40826).
4d, w, NetABΔGN/Y; sens-GAL4/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/TM2
Description: The three elements on the third chromosome use the STaR system to label ~70% of R8s with myr::GFP (see genotype description for 1e). sens-GAL4 is included as a genetic background control for the fra-Net epistasis experiment.
4e, w, NetABΔGN/Y; sens-GAL4/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/ UAS-fra RNAi (DRSC HMS01147)
Description: See genotype description for 4d.
4f, Net: Same as 4d.; Net+fraRNAi: Same as 4e
4g,H,i,
WT: w/Y; UAS-myr::tdTOM/Sp; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/R23G11-GAL4
Net: w, NetABΔGN/Y; UAS-myr::tdTOM/+; sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP/R23G11-GAL4
Description: The three elements on the third chromosome use the STaR system to label ~70% of R8s with myr::GFP (see genotype description for 1e). R23G11-GAL4 (RRID:BDSC_49043) drives expression of myr::tdTOM in Dm4 cells in the adult (Nern et al., 2015).
4j,
Dm1-WT: Similar to 4g-WT with R22D12-GAL4 (RRID:BDSC_48983) driving Dm1 specific expression (Nern et al., 2015).
Dm1-Net: Similar to 4g -Net with R22D12-GAL4 (RRID:BDSC_48983) driving Dm1 specific expression.
Dm6-WT: Similar to 4g -WT with R38H06-GAL4 (RRID:BDSC_50029) driving Dm6 specific expression (Nern et al., 2015).
Dm6-Net: Similar to 4g -Net with R38H06-GAL4 (RRID:BDSC_50029) driving Dm6 specific expression.
5a-c,f-j, ey3.5-FLP1/+; FRT42B, ACT-GAL80/FRT42B, fra3; sens-GAL4, UAS-myr::tdTOM/ sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP
Description: Mitotic recombination in the visual system in this MARCM (Lee and Luo, 1999) genotype was driven by FLP1 recombinase (Nern et al., 2011) under the control of the ey-3.5 promoter fragment (Bazigou et al., 2007). The pairing of the ey-3.5 promoter with this higher efficiency variant of FLP largely preserves its specificity in the eye disc; in ~15% of the optic lobes we also noted recombination in lamina and medulla cell precursors. In our analysis, we did not detect a subclass of fra R8s with alternate developmental progression, suggesting that the possible existence of sporadic fra mutants of other cell classes does not change our main conclusions. The ACT-GAL80 element is based on a new actin-derived pan-cell promoter (provided by Barret Pfeiffer, Rubin Lab, Janelia Farm Research Campus/HHMI). fra R8s are labeled with myr::tdTOM (not shown). STaR system is used to drive genotype-independent expression of myr::GFP in ~70% of R8s (see genotype description for 1d).
5d, same as 4b
5e, Data compiled from two MARCM genotypes:
ey3.5-FLP1/+; FRT42B, ACT-GAL80/FRT42B, fra3; sens-GAL4, UAS-myr::tdTOM/ sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP , and
ey3.5-FLP1/+; FRT42B, ACT-GAL80/FRT42B, fra3; sens-GAL4, UAS-UtrnCH::GFP/ sens-R::pest, GMR-RpdOUT-myr::tdTOM
6, w, NetABΔGN/Y; Sp-+/+; sens-R::pest, GMR-RpdOUT-myr::tdTOM/+
Description: R8-specific expression of myr::tdTOM achieved as in 1b.
7a, ey3.5-FLP1/+; ACT-GAL80, FRT40A/FRT40A; sens-GAL4, UAS-UtrnCH::GFP/ TM2
7b, ey3.5-FLP1/+; ACT-GAL80, FRT40A/Trim991, FRT40A; sens-GAL4, UAS-UtrnCH::GFP/ Ly
7c, ey3.5-FLP1/+; ACT-GAL80, FRT40A/FRT40A; sens-GAL4, UAS-UtrnCH::GFP/ UAS-fra RNAi (DRSC HMS01147)
7d, ey3.5-FLP1/+; ACT-GAL80, FRT40A/Trim991,FRT40A; sens-GAL4, UAS-UtrnCH::GFP/ UAS-fra RNAi (DRSC HMS01147)
7e, Trim9null: Same as 7b.; Trim9null + fraRNAi: Same as 7d.
7f, ey3.5-FLP1/+; ACT-GAL80, FRT40A/Trim991, FRT40A; sens-GAL4, UAS-myr::tdTOM/ sens-R::pest, brp-RpdOUT-V5-2A-LexA, LexAop-myr::GFP
Description: FRT40A variant of the MARCM genotype in 5a.
Figure supplements
Request a detailed protocolFigure1-F.s.1, Same as 1e.
Figure3-F.s.1, Same as 3.
Figure5-F.s.1a,d, Same as 4b.
Figure5-F.s.1c, Same as 5a.
Figure5-F.s.1b,e, Same as 5e.
Figure5-F.s.2, Same as 5a.
References
-
Netrins guide Drosophila commissural axons at short rangeNature Neuroscience 9:188–194.https://doi.org/10.1038/nn1625
-
Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophinCell Motility and the Cytoskeleton 64:822–832.https://doi.org/10.1002/cm.20226
-
MATLAB Central File ExchangeSurface Fitting using gridfit, MATLAB Central File Exchange, http://www.mathworks.com/matlabcentral/fileexchange/8998.
-
MATLAB Central File ExchangeSlm-shape Language Modeling, MATLAB Central File Exchange, http://www.mathworks.com/matlabcentral/fileexchange/24443.
-
The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivoThe Journal of Cell Biology 201:903–913.https://doi.org/10.1083/jcb.201301091
-
Expression of baculovirus P35 prevents cell death in DrosophilaDevelopment 120:2121–2129.
-
Glycosaminoglycan-Binding Properties and secondary Structure of the C-Terminus of Netrin-1Biochemical and Biophysical Research Communications 271:287–291.https://doi.org/10.1006/bbrc.2000.2583
-
Cytonemes as specialized signaling filopodiaDevelopment 141:729–736.https://doi.org/10.1242/dev.086223
-
MATLAB Central File ExchangeB-Spline Grid, Image and Point based Registration, MATLAB Central File Exchange, http://www.mathworks.com/matlabcentral/fileexchange/20057.
-
Netrin-1 attracts Axons through Fak-Dependent MechanotransductionJournal of Neuroscience 32:11574–11585.https://doi.org/10.1523/JNEUROSCI.0999-12.2012
-
ScanImage: flexible software for operating laser scanning microscopesBiomedical Engineering Online 2:13.https://doi.org/10.1186/1475-925X-2-13
-
Fiji: an open-source platform for biological-image analysisNature Methods 9:676–682.https://doi.org/10.1038/nmeth.2019
-
Trim-9 functions in the Unc-6/Unc-40 pathway to regulate ventral guidanceJournal of Genetics and Genomics 38:1–11.https://doi.org/10.1016/j.jcg.2010.12.004
-
The molecular Biology of Axon GuidanceScience 274:1123–1133.https://doi.org/10.1126/science.274.5290.1123
-
Unc-6 (netrin) stabilizes oscillatory clustering of the Unc-40 (DCC) receptor to orient polarityThe Journal of Cell Biology 206:619–633.https://doi.org/10.1083/jcb.201405026
-
A novel Netrin-1-sensitive mechanism promotes local Snare-mediated exocytosis during axon branchingThe Journal of Cell Biology 205:217–232.https://doi.org/10.1083/jcb.201311003
-
Unc-6 (netrin) orients the invasive membrane of the anchor cell in C. ElegansNature Cell Biology 11:183–189.https://doi.org/10.1038/ncb1825
Article and author information
Author details
Funding
Howard Hughes Medical Institute
- S Lawrence Zipursky
Damon Runyon Cancer Research Foundation
- Orkun Akin
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Joshua Trachtenberg for his help in designing and building our 2P microscope. We thank Maya Bader for her contributions to the identification and initial characterization of Trim9 as an R8 targeting determinant. We thank Gerry Rubin, Aljoscha Nern, and Barret Pfeiffer for providing flies containing various cell-type specific GAL4s and the new GAL80 constructs. We thank Margot E. Quinlan for the UAS-UtrnCH::GFP DNA and flies. We thank Thomas Kidd for the NetABΔGN fly line. We thank Kazuo Emoto for the Trim991, FRT40A fly line. We thank Iris Salecker for the NetB::TM fly line. For critical reading of the manuscript, we thank Samantha Butler, Thomas R Clandinin, Claude Desplan, Matthew Y Pecot, Margot E Quinlan, Wael Tadros, Liming Tan, and Joshua Trachtenberg. This work was supported by a postdoctoral research fellowship from the Damon Runyon Cancer Research Foundation to OA. SLZ is an investigator of the Howard Hughes Medical Institute.
Copyright
© 2016, Akin et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,152
- views
-
- 732
- downloads
-
- 74
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 74
- citations for umbrella DOI https://doi.org/10.7554/eLife.20762






