Scientists have developed a lung-on-chip model to study how the body responds to early tuberculosis (TB) infection, according to findings published today in eLife.
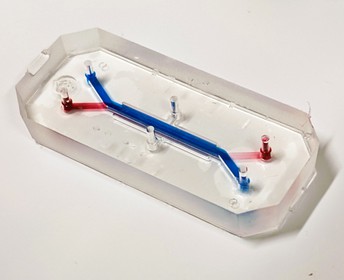
A close-up image of the lung-on-chip model. The endothelial or vascular channel is highlighted with a red food colouring dye, and the epithelial or airway channel is highlighted with a blue food colouring dye. The design allows for a co-culture of the cells from the two channels in the middle of the chip. Image credit: Vivek Thacker
(CC BY 4.0)
TB is a disease caused by the bacterium Mycobacterium tuberculosis (M. tuberculosis) and most often affects the lungs. The model reveals that respiratory system cells, called alveolar epithelial cells, play an essential role in controlling early TB infection. They do this by producing a substance called surfactant – a mixture of molecules (lipids and proteins) that reduce the surface tension where air and liquid meet in the lung.
These findings add to our understanding of what happens during early TB infection, and may explain in part why those who smoke or have compromised surfactant functionality have a higher risk of contracting primary or recurrent infection.
TB is one of the world’s top infectious killers and affects people of all ages. While it mostly affects adults, there are currently no effective vaccines available to this group. This is partly due to challenges with studying the early stages of infection, which take place when just one or two M. tuberculosis bacteria are deposited deep inside the lung.
“We created the lung-on-chip model as a way of studying some of these early events,” explains lead author Vivek Thacker, a postdoctoral researcher at the McKinney Lab, École polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland. “Previous studies have shown that components of surfactant produced by alveolar epithelial cells can impair bacterial growth, but that the alveolar epithelial cells themselves can allow intracellular bacterial growth. The roles of these cells in early infection are therefore not completely understood.
"We used our model to observe where the sites of first contact are, how M. tuberculosis grows in alveolar epithelial cells compared to bacteria-killing cells called macrophages, and how the production of surfactant affects growth, all while maintaining these cells at the air-liquid interface found in the lung.”
The team used their lung-on-chip model to recreate a deficiency in surfactant produced by alveolar epithelial cells and then see how the lung cells respond to early TB infection. The technology is optically transparent, meaning they could use an imaging technique called time-lapse microscopy to follow the growth of single M. tuberculosis bacteria in either macrophages or alveolar epithelial cells over multiple days.
Their studies revealed that a lack of surfactant results in uncontrolled and rapid bacterial growth in both macrophages and alveolar epithelial cells. On the other hand, the presence of surfactant significantly reduces this growth in both cells and, in some cases, prevents it altogether.
“Our work shines a light on the early events that take place during TB infection and provides a model for scientists to build on for future research into other respiratory infections,” says senior author John McKinney, Head of the Laboratory of Microbiology and Microtechnology at EPFL. “It also paves the way for experiments that increase the complexity of our model to help understand why some TB lesions progress while others heal, which can occur at the same time in the same patient. This knowledge could one day be harnessed to develop effective new interventions against TB and other diseases.”
The authors add that they are currently using a human lung-on-chip model to study how our lungs may respond to a low-dose infection and inoculation of SARS-CoV-2, the virus that causes COVID-19.
##
This study was originally posted on the preprint server bioRxiv, at https://www.biorxiv.org/content/10.1101/2020.02.03.931170v2.
Author contact for more information:
Vivek Thacker, postdoctoral researcher at EPFL
Email: vivek.thacker@epfl.ch
Twitter: @DrVivekThacker
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373
About
eLife is a non-profit organisation created by funders and led by researchers. Our mission is to accelerate discovery by operating a platform for research communication that encourages and recognises the most responsible behaviours. We work across three major areas: publishing, technology and research culture. We aim to publish work of the highest standards and importance in all areas of biology and medicine, including Microbiology and Infectious Disease, and the Physics of Living Systems, while exploring creative new ways to improve how research is assessed and published. We also invest in open-source technology innovation to modernise the infrastructure for science publishing and improve online tools for sharing, using and interacting with new results. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, the Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Microbiology and Infectious Disease research published in eLife, visit https://elifesciences.org/subjects/microbiology-infectious-disease.
And for the latest in the Physics of Living Systems, see https://elifesciences.org/subjects/physics-living-systems.




