Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorWeiwei DangBaylor College of Medicine, Houston, United States of America
- Senior EditorPankaj KapahiBuck Institute for Research on Aging, Novato, United States of America
Reviewer #1 (Public review):
Summary:
In this study, the authors showed that enalapril was able to reduce cellular senescence and improve health status in aged mice. The authors further showed that phosphorylated Smad1/5/9 was significantly elevated and blocking this pathway attenuated the protection of cells from senescence. When middle-aged mice were treated with enalapril, the physiological performance in several tissues, including memory capacity, renal function, and muscle strength, exhibited significant improvement.
Strengths:
The strength of the study lies in the identification of the pSMAD1/5/9 pathway as the underlying mechanism mediating the anti-senescence effects of enalapril with comprehensive evaluation both in vitro and in vivo.
Weaknesses:
The major weakness of the study is the in vivo data. Despite the evidence shown in the in vitro study, there is no data to show that blocking the pSmad1/5/9 pathway is able to attenuate the anti-aging effects of enalapril in the mice. In addition, the aging phenotypes mitigation by enalapril is not evidenced by the extension of lifespan. If it is necessary to show that NAC is able to attenuate enalapril effects in the aging mice. In addition, it would be beneficial to test if enalapril is able to achieve similar rescue in a premature aging mouse model.
Reviewer #2 (Public review):
This manuscript presents an interesting study of enalapril for its potential impact on senescence through the activation of Smad1/5/9 signaling with a focus on antioxidative gene expression. Repurposing enalapril in this context provides a fresh perspective on its effects beyond blood pressure regulation. The authors make a strong case for the importance of Smad1/5/9 in this process, and the inclusion of both in vitro and in vivo models adds value to the findings. Below, I have a few comments and suggestions which may help improve the manuscript.
A major finding in the study is that phosphorylated Smad1/5/9 mediates the effects of enalapril. However, the manuscript focused on the Smad pathway relatively abruptly, and the rationale behind targeting this specific pathway is not fully explained. What makes Smad1/5/9 particularly relevant to the context of this study?
Furthermore, their finding that activation of Smad1/5/9 leads to a reduction of senescence appears somewhat contradictory to the established literature on Smad1/5/9 in senescence. For instance, studies have shown that BMP4-induced senescence involves the activation of Smad1/5/8 (Smad1/5/9), leading to the upregulation of senescence markers like p16 and p21 (JBC, 2009, 284, 12153). Similarly, phosphorylated Smad1/5/8 has been shown to promote and maintain senescence in Ras-activated cells (PLOS Genetics, 2011, 7, e1002359). Could the authors provide more detailed mechanistic insights into why enalapril seems to reverse the typical pro-senescent role of Smad1/5/9 in their study?
While the authors showed that enalapril increases pSmad1/5/9 phosphorylation, what are the expression levels of other key and related factors like Smad4, pSmad2, pSmad3, BMP2, and BMP4 in both senescent and non-senescent cells? These data will help clarify the broader signaling effects.
They used BMP receptor inhibitor LDN193189 to pharmacologically inhibit BMP signaling, but it would be more convincing to also include genetic validation (e.g., knockdown or knockout of BMP2 or BMP4). This will help confirm that the observed effects are truly due to BMP-Smad signaling and not off-target effects of the pharmacological inhibitor LDN.
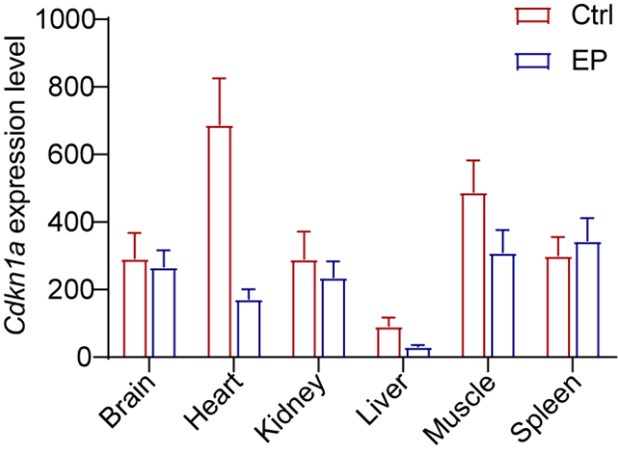
I don't see the results on the changes in senescence markers p16 and p21 in the mouse models treated with enalapril. Similarly, the effects of enalapril treatment on some key SASP factors, such as TNF-α, MCP-1, IL-1β, and IL-1α, are missing, particularly in serum and tissues. These are important data to evaluate the effect of enalapril on senescence.
Given that enalapril is primarily known as an antihypertensive, it would be helpful to include data on how it affects blood pressure in the aged mouse models, such as systolic and diastolic blood pressure. This will clarify whether the observed effects are independent of or influenced by changes in blood pressure.