Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
(1) As part of getting rid of cross-contamination in the bulk data, the authors model the scRNA data, extrapolate it to the bulk data and subtract out "contaminant" cell types. One wonders, however, given that low expressed genes are not represented in the scRNA data, whether the assignment of a gene to one or another cell type can really be made definitive. Indeed, it's possible that a gene is expressed at low levels in one cell, and high levels in another, and would therefore be considered a contaminant. The result would be to throw out genes that actually are expressed in a given cell type. The definitive list would therefore be a conservative estimate, and not necessarily the correct estimate.
We agree that the various strategies we employ do not result in perfect annotation of gene expression. However, despite their limitations, they are significantly better than either the single cell or the bulk data alone. We represent these strengths and shortcomings throughout the manuscript (for example, in ROC curves).
(2) It would be quite useful to have tested some genes with lower expression levels using in vivo gene-fusion reporters to assess whether the expression assignments hold up as predicted. i.e. provide another avenue of experimentation, non-computational, to confirm that the decontamination algorithm works.
We agree that evaluating only highly-expressed genes might introduce bias. We used a large battery of in vivo reporters, made with best-available technology (CRISPR insertion of the fluorophore into the endogenous locus) to evaluate our approaches. These reporters were constructed without bias in terms of gene expression and therefore represent both high and low expression levels. These data are represented throughout the manuscript (for example, in ROC curves). Details about the battery of reporters may be found in Taylor et al 2021. In addition to these reporters, this manuscript also generates and analyzes two other types of gene sets: non-neuronal and ubiquitous genes. Again, these genes are selected without bias toward gene expression, and the techniques presented here are benchmarked against them as well, with positive results.
(3) In many cases, each cell class would be composed of at least 2 if not more neurons. Is it possible that differences between members of a single class would be missed by applying the cleanup algorithms? Such transcripts would be represented only in a fraction of the cells isolated by scRNAseq, and might then be considered not real.
For the data set presented in this manuscript, all cells of a single neuron type were labeled and isolated together by FACS, and sequencing libraries were constructed from this pool of cells. Thus, potential subtypes within a particular type (when that type includes more than one cell) cannot be resolved by this method. By contrast, such subtypes were in some cases resolved in the single cell approach. To make the two data sets compatible with each other, for the single cell data we combined any subtypes together. We state in the Methods:
“For this work, single cell clusters of neuron subtypes were collapsed to the resolution of the bulk replicates (example: VB and VB1 clusters in the single cell data were treated as one VB cluster).”
(4) I didn't quite catch whether the precise staging of animals was matched between the bulk and scRNAseq datasets. Importantly, there are many genes whose expression is highly stage-specific or age-specific so even slight temporal differences might yield different sets of gene expression.
We agree that accurate staging is critically important for valid comparisons between data sets and have included an additional supplemental table with staging metadata for each sample. The staging protocol used for the bulk data set was initially employed to generate scRNA seq data and should be comparable. An additional description of our approach is now included in Methods:
“Populations of synchronized L1s were grown at 23 C until reaching the L4 stage on 150 mM 8P plates inoculated with Na22. The time in culture to reach the L4 stage varied (40.5-49 h) and was determined for each strain. 50-100 animals were inspected with a 40X DIC objective to determine developmental stage as scored by vulval morphology (Mok et al., 2025). Cultures were predominantly composed of L4 larvae but also typically included varying fractions of L3 larvae and adults.”
We have also updated supplementary table 1 to include additional information about each sort including the observed developmental stages and their proportions when available, the temperature the worms were grown at, the genotype of each experiment, and the number of cells collected in FACS.
(5) To what extent does FACS sorting affect gene expression? Can the authors provide some controls?
We appreciate this suggestion. We agree that FACS sorting (and also dissociation of the animals prior to sorting) might affect gene expression, particularly of stress-related transcripts. We note that dissociation and FACS sorting was also used to collect cells for our single cell data set (Taylor et al 2021). We would note that clean controls for this approach can be prohibitively difficult to collect, as the process of dissociation and FACS will inevitably change the proportion of cell types present in the sample, and for bulk sequencing efforts it is difficult even with deconvolution approaches to accurately account for changes in gene expression that result from dissociation and FACS, versus changes in gene expression that result from differences in cell type composition. We regrettably omitted a discussion of these issues in the manuscript. We now write in the Results:
“The dissociation and FACS steps used to isolate neuron types induce cellular stress responsive pathways (van den Brink et al., 2017; Kaletsky et al., 2016, Taylor 2021). Genes associated with this stress response (Taylor 2021) were not removed from downstream analyses, but should be viewed with caution.”
Reviewer #2 (Public review):
The bulk RNA-seq data collected by the authors has high levels of contamination and, in some cases, is based on very few cells. The methodology to remove contamination partly makes up for this shortcoming, but the high background levels of contaminating RNA in the FACS-isolated neurons limit the confidence in cell-specific transcripts.
We agree that these are the limitations of the source data. One of the manuscript’s main goals is to analyze and refine these source data, reducing these limitations and quantifying the results.
The study does not experimentally validate any of the refined gene expression predictions, which was one of the main strengths of the initial CenGEN publication (Taylor et al, 2021). No validation experiments (e.g., fluorescence reporters or single molecule FISH) were performed for protein-coding or non-coding genes, which makes it difficult for the reader to assess how much gene predictions are improved, other than for the gold standard set, which may have specific characteristics (e.g., bias toward high expression as they were primarily identified in fluorescence reporter experiments).
We agree that evaluating only highly-expressed genes might introduce bias. We used a large battery of in vivo reporters, made with best-available technology (CRISPR insertion of the fluorophore into the endogenous locus) to evaluate our approaches. These reporters were constructed without bias in terms of gene expression and therefore represent both high and low expression levels. These data are represented throughout the manuscript (for example, in ROC curves). Details about the battery of reporters may be found in Taylor et al 2021. In addition to these reporters, this manuscript also generates and analyzes two other types of gene sets: non-neuronal and ubiquitous genes. Again, these genes are selected without bias toward gene expression, and the techniques presented here are benchmarked against them as well, with positive results.
The study notes that bulk RNA-seq data, in contrast to scRNA-seq data, can be used to identify which isoforms are expressed in a given cell. However, no analysis or genome browser tracks were supplied in the study to take advantage of this important information. For the community, isoform-specific expression could guide the design of cell-specific expression constructs or for predictive modeling of gene expression based on machine learning.
We strongly agree that these datasets allow for new discoveries in neuronal splicing patterns and regulators, which is explored further in other publications from our group and other research groups in the field. We did not sufficiently highlight these works in the body of our text, and have added a reference in the discussion. “In addition, the bulk RNA-seq dataset contains transcript information across the gene body, which parallel efforts have used to identify mRNA splicing patterns that are not found in the scRNA-seq dataset.” These works can be found in references 26 and 27.
(1) The study relies on thresholding to determine whether a gene is expressed or not. While this is a common practice, the choice of threshold is not thoroughly justified. In particular, the choice of two uniform cutoffs across protein-encoding RNAs and of one distinct threshold for non-coding RNAs is somewhat arbitrary and has several limitations. This reviewer recommends the authors attempt to use adaptive threshold-methods that define gene expression thresholds on a per-gene basis. Some of these methods include GiniClust2, Brennecke's variance modeling, HVG in Seurat, BASiCS, and/or MAST Hurdle model for dropout correction.
We appreciate the reviewer’s suggestion, and would note that the integrated data currently incorporates some gene-specific weighting to identify gene expression patterns, as the single-cell data are weighted by maximum expression for each gene prior to integration with the LittleBites cleaned data. This gene level normalization markedly improved gene detection accuracy, and is discussed in depth in our 2021 Paper “Molecular topography of an entire nervous system”. We previously explored several methods for setting gene specific thresholds for identifying gene expression patterns in the integrated dataset. Unfortunately we found that none of the tested methods out performed setting “static” thresholds across all genes in the integrated dataset, and tended to increase false positive rates for some low abundance genes, where gene-specific thresholding can tend towards calling a gene expressed in at least one cell type when it is actually not expressed in any cell types present. These methods are likely to provide better results for expanded datasets that cover all tissue types (where one might reasonably expect that a gene is likely to be expressed in at least one sample).
(2) Most importantly, the study lacks independent experimental validation (e.g., qPCR, smFISH, or in situ hybridization) to confirm the expression of newly detected lowly expressed genes and non-coding RNAs. This is particularly important for validating novel neuronal non-coding RNAs, which are primarily inferred from computational approaches.
We agree that smFISH and related in situ validation methods would be an asset in this analysis. Unfortunately because most ncRNAs are very short, they are prohibitively difficult to accurately measure with smFISH. Many ncRNAs we attempted to assay with smFISH methods can bind at most 3 fluorescent probes, which unfortunately was not reliably distinguishable from background autofluorescence in the worm. Many published methods for smFISH signal amplification have not been optimized for C. elegans, and the tough cuticle is a major barrier for those efforts.
(3) The novel biology is somewhat limited. One potential area of exploration would be to look at cell-type specific alternative splicing events.
We appreciate this suggestion. Indeed, as we put our source data online prior to publishing this manuscript, two published papers already use this source data set to analyze alternative splicing. Further, these works include validation of splicing patterns observed in this source data, indicating the biological relevance of these data sets.
(4) The integration method disproportionately benefits neuron types with limited representation in scRNA-seq, meaning well-sampled neuron types may not show significant improvement. The authors should quantify the impact of this bias on the final dataset.
We agree that cell-types that are well represented in the single-cell dataset tend to have fewer new genes identified in the Integrated dataset than “rare” cell-types in the single cell data. However we would note that cell-types that are highly abundant in the single-cell data appear to become increasingly vulnerable to non-neuronal false positives, and that integration’s primary effect in high abundance cell-types appears to be reducing the false positive rate for non-neuronal genes. Thus we suggest that integration benefits all cell-types across the spectrum of single-cell abundance. The false positives are likely caused by a side-effect of normalization steps in the single-cell dataset, which is moderated by using the LittleBites cleaned bulk samples as an orthogonal measurement. The benefit of integration for cell-types with low abundance in the single-cell dataset is now quantified, and the benefits of integration for low and high abundance cell-types from the single cell data are described in the following section (p.13):
“To test the stability of LittleBites cleanup across different single-cell reference dataset qualities, we ran the algorithm on a set of bulk samples by first subsetting the corresponding single-cell cluster’s population to 10, 50, 100, or 500 cells. We performed this process 500 times for each subsampling rate for each sample (2000 total runs per sample). We found that testing gene AUROC values are stable across reference cluster sizes (Fig. 2D), suggesting that even if the target cell type is rarely represented in a single cell reference, accurate cleaning is still possible. However, comparing gene level stability across target cluster population levels reveals that low abundance references have higher gene level variance (Fig. 2E), lower purity estimates (Fig. S2F), higher variance in the mean expression across genes (Fig. S2G), and they tend to have lower overall expression (suggesting more aggressive subtraction) (Fig. S2H). This indicates that while binary gene calling is improved even if the reference cluster is small, users should be cautious when using fewer than 100 cells in their single cell reference cluster as the resulting cleanup is less stable.”
(5) The authors employ a logit transformation to model single-cell proportions into count space, but they need to clarify its assumptions and potential pitfalls (e.g., how it handles rare cell types).
We agree that the assumptions and pitfalls of the logit model are key for evaluating its usefulness, especially for cell types that are rarely captured in the single-cell dataset. The assumptions and pitfalls are described in the methods section, but we regretfully omitted any mention of those pitfalls in the results, which we have now rectified.
The description in the methods section is: “We applied this formula to our real single cell dataset and used this equation to transform proportion measures of gene expression into a count space to generate the Prop2Count dataset for downstream analysis and integration with bulk datasets. This procedure allows for proportions data to be used in downstream analyses that work with counts datasets. However, it does limit the range of potential values that each gene can have, with the potential values set as: 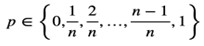
As n approaches 0, the number of potential values decreases, which can be incompatible with some downstream models. Thus, caution should be used when applying this transformation to datasets with few cells.”
The new mention in the results is: “However, caution should be taken when using this approach in scRNAseq cases where all replicates of a cell type contain few cells. scProp2Count values are limited to the space of possible proportion values, and so replicates with low numbers of cells will have fewer potential expression “levels” which may break some model assumptions in downstream applications (see Methods).”
(6) The LittleBites approach is highly dependent on the accuracy of existing single-cell references. If the scRNA-seq dataset is incomplete or contains classification biases, this could propagate errors into the bulk RNA-seq data. The authors may want to discuss potential limitations and sensitivity to errors in the single-cell dataset, and it is critical to define minimum quality parameters (e.g. via modeling) for the scRNAseq dataset used as reference.
We appreciate this suggestion, and agree that manuscript would benefit from a description of where the LittleBites method can give poor results. To this end, we subset our single cell reference for individual neurons of interest to the level of 10, 50, 100, or 500 cells (500 iterations per sample rate), and then ran Littlebites, and compared metrics of gene expression stability, sample composition estimates, and AUROC performance on test genes. We found that when fewer than 100 cells for the target cell type are included in the single cell reference, gene expression stability drops (variance between subsampling iterations was much higher when fewer reference cells were used). However, we found that AUROC values were consistently high regardless of how many reference cells were included, but that this stability in AUROC values was paired with lower overall counts in samples with <100 reference cells after cleanup. This indicates that in cases where few reference cells are present, higher AUROC values might be achieved by more aggressive subtraction, which is attenuated when the reference model is more complete. This analysis is shown in figure 2 and figure S2, and described in the results section, recreated here.
“To test the stability of Littlebites cleanup across different single-cell reference dataset qualities, we ran the algorithm on a set of bulk samples by first subsetting the corresponding single-cell cluster’s population to 10, 50, 100, or 500 cells. We performed this process 500 times for each subsampling rate for each sample (2000 total runs per sample). We found that testing gene AUROC values are stable across reference cluster sizes (Fig. 2D), suggesting that even if the target cell type is rarely represented in a single cell reference, accurate cleaning is still possible. However, comparing gene level stability across target cluster population levels reveals that low population references have higher gene level variance (Fig. 2E), lower purity estimates (Fig. S2F), higher variance in the mean expression across genes (Fig. S2G), and they tend to have lower overall expression (suggesting more aggressive subtraction) (Fig. S2H). This suggests that while binary gene calling is improved similarly even if the reference cluster is small, users should be cautious when using less than 100 cells in their single cell reference cluster as the resulting cleanup is less stable.”
(7) Also very important, the LittleBites method could benefit from a more intuitive explanation and schematic to improve accessibility for non-computational readers. A supplementary step-by-step breakdown of the subtraction process would be useful.
We appreciate this suggestion and implemented a step-by-steo breakdown of the subtraction process in the methods section, also copied below. We also updated the graphic representation in figure 2A.
“LittleBites Subtraction algorithm
LittleBites is an iterative algorithm for bulk RNA-seq datasets, that improves the accuracy of cell-type specific bulk RNA-seq sample profiles by removing counts from non-target contaminants (e.g. ambient RNA from dead cells, carry-over non-target cells from FACS enrichment due to imperfect gating). This method leverages single cell reference datasets and ground truth expression information to guide iterative and conservative subtraction to enrich for true target cell-type expression. Using this approach, LittleBites balances subtraction by optimizing using both a single-cell reference, and an orthogonal ground truth reference, moderating biases inherent to either reference.
This algorithm first calculates gene level specificity weights in a single cell reference dataset using SPM (Specificity Preservation Method) (re-add 22, re-add 23). SPM assigns high weights (approaching 1) to genes expressed in single cell types while applying conservative weights to genes with broader expression patterns, which helps to reduce inappropriate subtraction.
The algorithm proceeds in a loop of three steps:
Step 1: Estimate Contamination. Each bulk sample is modeled as the sum of a linear combination of single-cell profiles (target cell type and likely contaminants) using non-negative least squares (NNLS). The resulting coefficients provide the estimate of how much of the sample’s counts come from the target cell-type, and how much comes from each contaminant cell-type.
Step 2: Weighted Subtraction. Each bulk sample is cleaned by subtracting the weighted sum of contaminant single-cell profiles. This subtraction is attempted multiple times (separately) across a series of learning rate weights (usually ranging from 0-1) which moderate the size of the subtraction step (Equation 1). This produces a range of possible “cleaned” sample options for evaluation.
Step 3: Performance Optimization. For each learning rate, the cleaned result is evaluated against a set of ground truth genes by calculating the area under the receiver operating characteristic curve (AUROC). The learning rate that optimizes the AUROC is then selected. When multiple learning rates yielded equivalent AUROC values, the lowest learning rate value is chosen to minimize subtraction.
If the optimal learning rate at Step 3 is 0 (no subtraction option beats the baseline) then the loop is halted. Else, the cleaned bulk profile is returned to Step 1, and the loop continues until the AUROC cannot be improved upon using the single-cell reference modeling.“
(8) In the same vein, the ROC curves and AUROC comparisons should have clearer annotations to make results more interpretable for readers unfamiliar with these metrics.
We agree that the ROC and AUROC metrics need a clearer explanation to make their use and interpretations clearer. We included a description of both metrics, and a suggestion for how to interpret them in the results section, copied below.
“To evaluate the post-subtraction datasets accuracy we used the area under the Receiver-Operator Characteristic (AUROC) score. In brief, we set a wide range of thresholds to call genes expressed or unexpressed, and then compared it to expected expression from a set of ground truth genes. This comparison produces a true positive rate (TPR, the percentage of truly expressed genes that are called expressed), and false positive rate (FPR, the percentage of truly not expressed genes that are called expressed), and a false discovery rate (FDR, the percentage of genes called expressed that are truly not expressed). The Receiver-Operator Characteristic (ROC) is the graph of the line produced by the TPR and FPR values across the range of thresholds tested, and the AUROC is calculated as the sum of the area under that line. A “random” model of gene expression is expected to have an AUROC value of 0.5, and a “perfect” model is expected to have an AUROC value of 1. Thus, AUROCs below 0.5 are worse than a random guess, and values closer to 1 indicate higher accuracy.”
(9) Finally, after the correlation-based decontamination of the 4,440 'unexpressed' genes, how many were ultimately discarded as non-neuronal?
a) Among these non-neuronal genes, how many were actually known neuronal genes or components of neuronal pathways (e.g., genes involved in serotonin synthesis, synaptic function, or axon guidance)?
b) Conversely, among the "unexpressed" genes classified as neuronal, how many were likely not neuron-specific (e.g., housekeeping genes) or even clearly non-neuronal (e.g., myosin or other muscle-specific markers)?
Combined with point 10, see below.
(10) To increase transparency and allow readers to probe false positives and false negatives, I suggest the inclusion of:
a) The full list of all 4,440 'unexpressed' genes and their classification at each refinement step. In that list flag the subsets of genes potentially misclassified, including:
- Neuronal genes wrongly discarded as non-neuronal.
- Non-neuronal genes wrongly retained as neuronal.
b) Add a certainty or likelihood ranking that quantifies confidence in each classification decision, helping readers validate neuronal vs. non-neuronal RNA assignments.
This addition would enhance transparency, reproducibility, and community engagement, ensuring that key neuronal genes are not erroneously discarded while minimizing false positives from contaminant-derived transcripts.
We agree that the genes called “unexpressed” in the single-cell data need more context and clarity. First, we trimmed the list to only include 2,333 genes of highest confidence. Second, for those genes we identified any with published neuronal expression patterns. Identifying genes that were retained as neuronal but are likely non-neuronal in origin is difficult as many markers are expressed in a mixture of neuronal and non-neuronal cell-types, however we used a curated list of putative non-neuronal markers to assess the accuracy of the integrated data (see supplementary table 4), and established that most non-neuronal markers are undetected in the integrated data, with the number of detected genes decreasing as our threshold stringency increases. Of note, a few putative non-neuronal genes remain detected even at high thresholds, indicating that our dataset still retains a small percentage of neuronal false positives. This result has been collected in the new supplementary figure 4F, and addressed in the following text in the results section “Testing against a curated list of non-neuronal genes from fluorescent reporters and genomic enrichment studies, we found that of 445 non-neuronal markers, each gene was detected in an average of 12.5 cells or a median of 3 cells in the single-cell dataset, and an average of 8.7 cells or a median of 1 cell in the integrated dataset, at a 14% FDR threshold.”
We also included a list of “unexpressed” gene identities and tissue annotations as new supplementary tables 16 and 17.
Reviewer #2 (Recommendations for the authors):
The utility of the bulk RNA-seq data would be significantly increased if the authors were to analyze which isoforms are expressed in individual neurons. Also, it would be very useful to know if there are instances where a gene is expressed in several neurons, but different isoforms are specific to individual neurons.
We appreciate this suggestion. Indeed, as we put our source data online prior to publishing this manuscript, two published papers already use this source data set to analyze alternative splicing. Further, these works include validation of splicing patterns observed in this source data, indicating the biological relevance of these data sets. This is now noted in our discussion section “In addition, the bulk RNA-seq dataset contains transcript information across the gene body, which parallel efforts have used to identify mRNA splicing patterns that are not found in the scRNA-seq dataset.” These works can be found in references 26 and 27.
Reviewer #3 (Recommendations for the authors):
(1) Describe the number of L4 animals processed to obtain good-quality bulk RNAseq libraries from the different neuronal types. If the number of worms would be different for different neuronal types, then please make a supplementary table listing the minimum number of worms needed for each neuronal type.
We appreciate the reviewer’s recommendation, and agree that it would be a useful resource to provide suggestions for how many worms are needed per experiment. Unfortunately We did not track the total number of animals for each sample. We aimed to start with 200-300 ul of packed worms for each strain, generally equating to >500,000 worms, but yields of FACS-isolated cells varied among cell types. Because replicates for specific neuron types were also variable in some instances (See additions to supplemental Table 1), yields likely depend on multiple factors. We have previously noted (Taylor et al., 2021), for example, that some cell types were under-represented in scRNA-seq data (e.g, pharyngeal neurons) based on in vivo abundance presumptively due to the difficulty of isolation or sub-viability in the cell dissociation-FACS protocol.
(2) List the thresholds for the parameters used during the FASTQC quality control and the threshold number of reads that would make a sample not useful.
We now include parameters for sample exclusion in the methods section. “Samples were excluded after sequencing if they had: fewer than 1 million read pairs, <1% of uniquely mapping reads to the C. elegans genome, > 50% duplicate reads (low umi diversity), or failed deduplication steps in the nudup package.”
(3) In Figure 5C, include an overlapping bar that shows the total number of genes in each cell type. You may need to use a log scale to see both (new and all) numbers of genes in the same graph. Add supplementary tables with the names of all new genes assigned to each neuronal type.
We agree that this figure panel needed additional context. On further reflection we concluded that figure 5 was not sufficiently distinct from figure 4 to warrant separation, and incorporated some key findings from figure 5 into figure S4.