Author Response
The following is the authors’ response to the original reviews.
Reviewer 1 (public):
- “It is unclear whether new in vivo experiments were conducted for this study”.
All in vivo experiments were conducted for this study by using previously published fly stocks to directly compare N- and C-terminal shedding side-by-side in two Hh-dependent developmental systems. This is now clearly stated in the revised supplement (Fig. S8). We also conducted these experiments because previous in vivo studies in flies often relied on Hh overexpression in the fat body, raising questions about their physiological relevance. Our in vivo analyses of Hh function in wing and eye discs are more physiologically relevant and can explain the previously reported presence of non-lipidated bioactive Hh in disc tissue (PMID: 23554573).
- “A critical shortcoming of the study is that experiments showing Shh secretion/export do not include a Shh(-) control condition. Without demonstration that the bands analyzed are specific for Shh(+) conditions, these experiments cannot be appropriately evaluated”.
The Cell Signaling Technology C9C5 anti-Shh antibody used in our study is highly specific against Shh, and it has been used in over 60 publications. C9C5 even lacks cross-reactivity with highly similar Ihh or Dhh (https://www.cellsignal.com/products/primary-antibodies/shh-c9c5-rabbit-mab/2207?_requestid=1528451). We confirmed C9C5 specificity repeatedly (one example is shown below; another quality control that includes media of mock-transfected cells is now shown in Fig. S1) and never observed unspecific bands under any experimental condition. As shown below, C9C5 and R&D AF464 anti-Shh antibodies (the latter were previously used in our lab) detect the same bands.
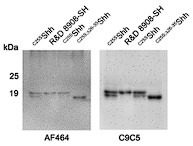
Author response image 1.
Shh immunoblot. R&D 8908-SH served as a size control for full-length dual-lipidated Shh, and C25S;26-35Shh served as a size control for N-terminally truncated monolipidated Shh. Both C25SShh bands are specific: One represents the full-length protein and the bottom band represents N-truncated processed proteins. The blot was first incubated with antibody AF464 and reincubated (after stripping) with the much more sensitive antibody C9C5.
- “A stably expressing Shh/Hhat cell line would reduce condition to condition and experiment to experiment variability”.
We agree and therefore have previously aimed to establish stable Hhat-expressing cell lines. However, we found that long-term Hhat overexpression eliminated transfected cells after several passages, or cells gradually ceased to express Hhat. This prevented us from establishing stable cell lines co-expressing Shh/Hhat despite several attempts and different strategies. Instead, we established transient co-expression of Shh/Hhat from the same mRNA as the next-best strategy for reliable near-quantitative Shh palmitoylation in our assays.
- “Unusual normalization strategies are used for many experiments, and quantification/statistical analyses are missing for several experiments”.
We repeated all qPCR assays to eliminate this shortcoming. Biological activities and transcriptional responses of palmitoylated Shh and non-palmitoylated C25AShh are now directly compared and quantified (revised Fig. 4A,B, newly included Fig. 6, revised Fig. S5B). The original comparison of both proteins with dual-lipidated R&D 8908-SH is still important in order to show that both Shh and C25AShh in serum-containing media have equally high, and not equally low, activities because R&D 8908-SH is generally seen as the Shh form with the highest biological activity. These comparisons are therefore still discussed in the main manuscript text and are now shown in Fig. S5E.
- “The study provides a modest advance in the understanding of the complex issue of Shh membrane extraction”
We believe that the revised manuscript advances our understanding of Shh membrane extraction beyond the modest in three important ways. First, although Disp was indeed known as a furin-activated Hh exporter, our findings show for the first time that furin activation of Disp is strictly linked to proteolytic Shh processing as the underlying release mode, fully consistent with data obtained from the Disp-/- cells.
Second, Scube2 was known as a Shh release enhancer and several lipoproteins were previously shown to play a role in the process, but our findings are the first to show that synergistic Disp/Scube2 function depends on the presence of lipoprotein and that HDL (but no other lipoprotein) accepts free cholesterol or a novel monolipidated Shh variant from Disp. This challenges the dominant model of Scube2 chaperone function in Hh release and transport (PMID 22902404, PMID 22677548, PMID 36932157).
Third, we show that this Shh variant is fully bioactive, despite the lack of the palmitate. Therefore, N-palmitate is dispensable for Shh signaling to Ptch1 receptors, but only if the morphogen is released by, and physically linked to, HDL. In contrast, previously published studies analyzed monolipidated Shh variants in the absence of HDL, resulting in variably reduced bioactivity of these physiologically irrelevant forms. Therefore, our findings challenge the current dominating model of N-palmitate-dependent Shh signaling to Ptch1 (this model also does not postulate any role for lipoproteins, PMID 36932157) and essential roles of N-palmitate (stating that the N-palmitate is sufficient for signaling, PMID 27647915).
Reviewer 2 (public):
- “However, the results concerning the roles of lipoproteins and Shh lipid modifications are largely confirmatory of previous results, and molecular identity/physiological relevance of the newly identified Shh variant remain unclear”.
We disagree with this assessment on several points. First, our findings do not confirm, but strongly challenge, the current dogma of Disp-mediated handover of dual-lipidated Shh to Scube2 as a soluble acceptor (instead of to HDL, PMID 36932157). Second, we report three new findings: Disp, Scube2, and lipoproteins all interact to specifically increase N-terminal Shh shedding, whereas C-terminal shedding is optional; Disp function depends on the presence of HDL; and HDL modulates Shh shedding (dual Shh shedding in the absence of HDL versus N-shedding and HDL association in its presence). Our work also directly determines the molecular identity of a previously unknown Shh variant as monolipidated (by RP-HPLC), HDL associated (by SEC and density gradient centrifugation), and fully bioactive (in two cell-based reporter assays).
Third, regarding the physiological relevance of our findings: Fig. S8 demonstrates that deletion of the N-terminal sheddase target site of Hh abolishes all Hh biofunction in Drosophila eye discs and wing discs, which strongly supports physiological relevance of N-terminal Hh shedding during release. N-terminal shedding is further consistent with in vivo findings of others. These studies showed that artificial monolipidated Shh variants (C25SShh and ShhN) generate highly variable loss-of-function phenotypes in vivo, but can also generate gain-of-function phenotypes if compared with the dual-lipidated cellular protein 1, 2, 3, 4, 5. These observations are difficult to align with the dominating model of essential N-palmitate function at the level of Ptch1 (PMID 36932157), because the lack of N-palmitate is expected to always diminish signaling in all tissue contexts and developmental stages. Our finding that dual-lipidated Shh is strictly released in a Disp/Scube2-controlled manner from producing cells, while artificial monolipidated Shh variants leak uncontrolled from the cellular surface, explains these seemingly paradoxical in vivo findings much better. This is because uncontrolled Shh release can increase Shh signaling locally (when physiological release would normally be prevented at this site 6 or time), while it can also decrease it (for example, in situations requiring timed pulses of Shh release and signaling 7, 8, 9, 10, 11). This is discussed in our manuscript (Discussion, first paragraph).
- The molecular properties of the processed Shh variants are unclear – incorporation of cholesterol/palmitate and removal of peptides were not directly demonstrated…
We also disagree on this point. Our study is the only one that uses RP-HPLC and defined controls (dual-lipidated commercial R&D 9808-SH, dual-lipidated cellular proteins eluting at the same positions, non-lipidated or monolipidated controls, Fig. S1F-K) to compare the lipidation status of cellular and corresponding solubilized Shh and to determine their exact lipidation status (Figs. 1, 3, 5, Figs. S4, S6, S7). Co-expressed Hhat assures full Shh palmitoylation during biosynthesis (as shown in original Figs. 1A and S2F-K & S4A and as confirmed by R&D 9808-SH) as an essential prerequisite to reliably conduct and interpret these analyses. The removal of peptides is demonstrated by the increase in electrophoretic mobility of soluble forms, if compared with their dual-lipidated cellular precursor, because chemical delipidation results in a decrease in electrophoretic mobility in SDS-PAGE (as discussed in detail in 12 that we now cite in our work).
- This (N-terminal palmitoylation status) is particularly relevant …, as the signaling activity of non-palmitoylated Hedgehog proteins is controversial.
We agree with this comment and are aware of the published data. However, in our work, we have demonstrated strong signaling activities by using C25AShh mutants that are fully impaired in their ability to undergo N-palmitoylation (Fig. 4, Fig. S5). These are highly bioactive if associated with HDL. Therefore, we do not see any ambiguity in our findings and suggest that the reports of others resulted from different experimental conditions.
- A decrease in hydrophobicity is no proof for cleavage of palmitate, this could also be due to addition of a shorter acyl group.
As shown in the original manuscript, we have controlled for this possibility: RP-HPLC was established by using defined controls (dual-lipidated, non-lipidated, or monolipidated, Fig. S1F-K and corresponding color coding). Because the cellular Shh precursor prior to release was always dual-lipidated, whereas the soluble form was not, lipids were clearly lost during release (because a decrease in the hydrophobicity of soluble proteins is always shown relative to that in their dual-lipidated cellular precursors). The increase in electrophoretic mobility detected for the very same proteins in SDS-PAGE demonstrates delipidation during their release (please see my reply to point 2 above). Finally, the suggested possibility of palmitate exchange for shorter acyls during Shh release at the cell surface is extremely unlikely, as there is no known machinery to catalyze this exchange at the plasma membrane. Hh acylation only occurs in the ER membrane via Hhat 13.
- “It would be important to demonstrate key findings in cells that secrete Shh endogenously”.
We now show that Panc1 cells release endogenous Shh in truncated form, as our transfected cells do (Fig. S1). Moreover, the experimental data shown in Fig. S8B demonstrate that engrailed-controlled expression of sheddase-resistant Hh variants in wing disc cells completely blocks endogenous Hh produced in the same cells by stalling Disp-mediated morphogen export. Both findings strongly support our key finding that N-processing is not optional but absolutely required to finalize Hh release.
- Co-fractionation of Shh and ApoA1 is not convincing, as the two proteins peak at different molecular weights…. The authors could use an orthogonal approach, optimally a demonstration of physical interaction, or at least fractionation by a different parameter
Shifted Shh peaks upon physiologically relevant Shh transfer via Disp to HDL must be expected in SEC, because Shh association with HDL subfractions increases their size. Comparing relative peaks of Shh-loaded HDL with Shh-free reference HDL suggests 10-15 Shh molecules per HDL (adding 200kDa - 300kDa to its molecular mass). This is now stated in the revised manuscript (page 10, line 2).
Still, to further support direct Shh/HDL association, we analyzed high molecular weight Shh SEC fractions by subsequent RP-HPLC. This approach confirms direct physical interactions between cholesteroylated Shh and HDL (now shown in Fig. S6G).
We support this possibility further by density gradient centrifugation, again demonstrating that Shh and HDL interact physically (now shown in Fig. S6 E,F).
Recommendations from the reviewing editor:
- “The authors should certainly tone down statements of novelty because much of the work is confirmatory in nature”
We followed this request in our revised manuscript and now clearly point out what was known and what we add to the concept of Disp and lipoprotein-mediated Hh export. Still, as outlined in our response to reviewer 2, our findings align with only one previously published model of lipoprotein-mediated Hh transport, while they do not support the most current models of Disp-mediated handover of dual-lipidated Shh to Scube2 (PMID 36932157) and essential signaling roles of N-palmitate at the level of the receptor Ptch1. Thus, our work should not be viewed solely as confirmatory of one of the many previous models, because at the same time it also contradicts the other models of Hh solubilization and transport.
- “Inclusion of the Shh(-) control”
Please see our reply to reviewer 1 above. The Cell Signaling Technology C9C5 anti-Shh antibody used in our study is highly specific against Shh. We also carefully characterized the C9C5 antibody before any of the experiments shown in our work had been initiated. We never observed any unspecific C9C5 reactivity that otherwise would – of course – have prevented us from switching to this antibody from the AF464 antibodies that we had previously used. Consistent C9C5 antibody specificity is evident from the representative example shown below that was recently produced in our lab: no cellular proteins or TCA-precipitated serum-depleted media components from mock-transfected cells (left two lanes) react with C9C5.
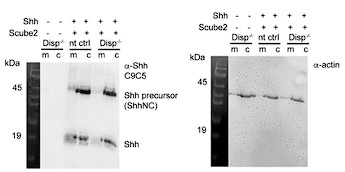
Author response image 2.
Top left: C9C5 detects the cellular 45kDa Shh precursor and the 19 kDa signaling-active protein. No unspecific signals are detected in untransfected cells and supernatants of such cells (left two lanes). Right: Loading control on the stripped blot.
- “Clean up how the data are normalized for quantification”
Please see our reply to reviewer 1 above. Normalization has been changed for the indicated figures. We also repeated qPCR analyses and added new ones to the manuscript that include required controls. We also changed figure outlines in accordance with the request.
- “The issue of a non-specific band of this Shh antibody is critical”
Please see our replies above. In our hands, unspecific C9C5 antibody binding was never observed.
- “Regarding experimental rigor, I would add that the HPLC … should just show the real data points”
We agree and added individual data points to our revised manuscript.
Recommendations for the authors:
- I would like to see the controls in the same figure with the experimental results.
We show antibody specificity controls together with released Shh in Fig. S1.
- Figure 2 confirms previously published results. It was shown in PMC5811216 that Disp processing by furin is required for Shh release from producing cells.
Indeed, it was shown that furin processing of Disp increases Shh release (supposedly together with lipids), but we show here that furin-activated Disp specifically mediates proteolytic Shh shedding and loss of lipids – which is not the same. Indeed, we show this finding because we interpret it the other way around: Because it is known that furin activation of Disp increases Shh release by some means (PMC5811216), our observation that furin-mediated Disp activation specifically increases Shh shedding independently supports our model.
- Figure 3: it is stated that there is no increase in Shh release into the media…
We removed this statement.
- Figure S5: Scale bars are missing.
We added scale bars to the figures.
- Figure 4: A direct comparison between wt Shh and C25A conditioned media for qPCR is needed.
We agree and repeated all experiments. Results confirm our previous findings and are shown in revised Fig. 4 and in Fig. S5.
- What other components can be examined in addition to ApoA1 as a marker for HDL? Why is the Shh peak shifted to the left? What about exovesicles?
We also detected ApoE4, a mobile lipoprotein present on expanding (large) HDL (Figs. 5, 6, Figs S6, 7) 14. We also used density gradient centrifugation to support the Shh/HDL association. Regarding the leftwards Shh size shift relative to the major HDL peak in SEC, please refer to our explanation above – if loaded with Shh, a size increase of the respective HDL subfraction is expected. Finally, we did not test the role of exovesicles in our assays. However, due to their large size (60-120nm, HDL 7-12 nm), Shh associated with exovesicles should have eluted in the void volume of our gel filtration column. This we never observed.
- Why is osteoblast differentiation used?
C3H10T1/2 osteoblast differentiation is strongly driven by Ihh and Shh activity and is established as a sensitive and robust assay. Still, following this reviewer’s advice, we conducted qPCR assays on these cells and in addition on NIH3T3 cells to support our findings.
Finally, we corrected all minor mistakes regarding spelling and figure labeling. We also improved the readability of the revised manuscript, as suggested by reviewer 2.
References
Gallet A, Ruel L, Staccini-Lavenant L, Therond PP. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development 133, 407-418 (2006).
Porter JA, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 86, 21-34 (1996).
Lewis PM, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599-612 (2001).
Huang X, Litingtung Y, Chiang C. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development 134, 2095-2105 (2007).
Lee JD, et al. An acylatable residue of Hedgehog is differentially required in Drosophila and mouse limb development. Dev Biol 233, 122-136 (2001).
Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131, 5581-5590 (2004).
Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest 114, 485-494 (2004).
Dessaud E, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720 (2007).
Garcia-Morales D, Navarro T, Iannini A, Pereira PS, Miguez DG, Casares F. Dynamic Hh signalling can generate temporal information during tissue patterning. Development 146, (2019).
Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528 (2004).
Nahmad M, Stathopoulos A. Dynamic interpretation of hedgehog signaling in the Drosophila wing disc. PLoS Biol 7, e1000202 (2009).
Ehring K, et al. Conserved cholesterol-related activities of Dispatched 1 drive Sonic hedgehog shedding from the cell membrane. J Cell Sci 135, (2022).
Coupland CE, et al. Structure, mechanism, and inhibition of Hedgehog acyltransferase. Mol Cell 81, 5025-5038 e5010 (2021).
Sacks FM, Jensen MK. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arterioscler Thromb Vasc Biol 38, 487-499 (2018).





