Author Response
eLife assessment
In this valuable study, the authors investigate the mechanism of amyloid nucleation in a cellular system using their novel ratiometric measurements and uncover interesting insights regarding the role of polyglutamine length and the sequence features of glutamine-rich regions on amyloid formation. Overall, the problem is significant and being able to assess nucleation in cells is of considerable relevance. The data, as presented and analyzed, are currently still incomplete. The specific claims would be stronger if based on in vitro measurements that avoid the intricacies of specific cellular systems and that are more suitable for assessing sequence-intrinsic properties.
We are pleased that the editors find our study valuable. We find that the reviewers’ criticisms largely arise from misunderstandings inherent to the conceptually challenging nature of the topic, rather than fundamental flaws, as we will elaborate here. We are grateful for the opportunity afforded by eLife to engage reviewers in a constructive public dialogue.
Reviewer #1 (Public Review):
The authors take on the challenge of defining the core nucleus for amyloid formation by polyglutamine tracts. This rests on the assertion that polyQ forms amyloid structures to the exclusion of all other forms of solids. Using their unique assay, deployed in yeast, the authors attempt to infer the size of the nucleus that templates amyloid formation by polyQ. Further, through a series of sequence titrations, all studied using a single type of assay, the authors converge on an assertion stating that a single polyQ molecule is the nucleus for amyloid formation, that 12-residues make up the core of the nucleus, that it takes ca. 60 Qs in a row to unmask this nucleation potential, and that polyQ amyloid formation belongs to the same universality class as self-poisoned crystallization, which is the hallmark of crystallization from polymer melts formed by large, high molecular weight synthetic polymers. Unfortunately, the authors have decided to lean in hard on their assertions without a critical assessment of whether their findings stand up to scrutiny. If their findings are truly an intrinsic property of polyQ molecules, then their findings should be reconstituted in vitro. Unfortunately, careful and rigorous experiments in vitro show that there is a threshold concentration for forming fibrillar solids. This threshold concentration depends on the flanking sequence context on temperature and on solution conditions. The existence of a threshold concentration defies the expectation of a monomer nucleus. The findings disagree with in vitro data presented by Crick et al., and ignored by the authors. Please see: https://doi.org/10.1073/pnas.1320626110. These reports present data from very different assays, the importance of which was underscored first by Regina Murphy and colleagues. The work of Crick et al., provides a detailed thermodynamic framework - see the SI Appendix. This framework dove tails with theory and simulations of Zhang and Muthukumar, which explains exactly how a system like polyQ might work (https://doi.org/10.1063/1.3050295). The picture one paints is radically different from what the authors converge upon. One is inclined to lean toward data that are gleaned using multiple methods in vitro because the test tube does not have all the confounding effects of a cellular milieu, especially when it comes to focusing on sequence-intrinsic conformational transitions of a protein. In addition to concerns about the limitations of the DAmFRET method, which based on the work of the authors in their collaborative paper by Posey et al., are being stretched to the limit, there is the real possibility that the cellular milieu, unique to the system being studied, is enabling transitions that are not necessarily intrinsic to the sequence alone. A nod in this direction is the work of Marc Diamond, which showed that having stabilized the amyloid form of Tau through coacervation, there is a large barrier that limits the loss of amyloid-like structure for Tau. There may well be something similar going on with the polyQ system. If the authors could show that their data are achievable in vitro without anything but physiological buffers one would have more confidence in a model that appears to contradict basic physical principles of how homopolymers self-assemble. Absent such additional evidence, numerous statements seem to be too strong. There are also several claims that are difficult to understand or appreciate.
Rebuttal to the perceived necessity of in vitro experiments
The overarching concern of this reviewer and reviewing editor is whether in-cell assays can inform on sequence-intrinsic properties. We understand this concern. We believe however that the relative merit of in-cell assays is largely a matter of perspective. The truly sequence-intrinsic behavior of polyQ, i.e. in a vacuum, is less informative than the “sequence-intrinsic” behaviors of interest that emerge in the presence of extraneous molecules from the appropriate biological context. In vitro experiments typically include a tiny number of these -- water, ions, and sometimes a crowding agent meant to approximate everything else. Obviously missing are the myriad quinary interactions with other proteins that collectively round out the physiological solvent. The question is what experimental context best approximates that of a living human neuron under which the pathological sequence-dependent properties of polyQ manifest. We submit that a living yeast cell comes closer to that ideal than does buffer in a test tube.
The reviewer’s statements that our findings must be validated in vitro ignores the fact -- stressed in our introduction -- that decades of in vitro work have not yet generated definitive evidence for or against any specific nucleus model. In addition to the above, one major problem concerns the large sizes of in vitro systems that obscure the effects of primary nucleation. For example, a typical in vitro experimental volume of e.g. 1.5 ml is over one billion-fold larger than the femtoliter volume of a cell. This means that any nucleation-limited kinetics of relevant amyloid formation are lost, and any alternative amyloid polymorphs that have a kinetic growth advantage -- even if they nucleate at only a fraction the rate of relevant amyloid -- will tend to dominate the system (Buell, 2017). Novel approaches are clearly needed to address these problems. We present such an approach, stretch it to the limit (as the reviewer notes) across multiple complementary experiments, and arrive at a novel finding that is fully and uniquely consistent with all of our own data as well as the collective prior literature.
That the preceding considerations are collectively essential to understand relevant amyloid behavior is evident from recent cryoEM studies showing that in vitro-generated amyloid structures generally differ from those in patients (Arseni et al., 2022; Bansal et al., 2021; Radamaker et al., 2021; Schmidt et al., 2019; Schweighauser et al., 2020; Yang et al., 2022). This is highly relevant to the present discourse because each amyloid structure is thought to emanate from a different nucleating structure. This means that in vitro experiments have broadly missed the mark in terms of the relevant thermodynamic parameters that govern disease onset and progression. Note that the rules laid out via our studies are not only consistent with structural features of polyQ amyloid in cells, but also (as described in the discussion) explain why the endogenous structure of a physiologically relevant Q zipper amyloid differs from that of polyQ.
A recent collaboration between the Morimoto and Knowles groups (Sinnige et al.) investigated the kinetics of aggregation by Q40-YFP expressed in C. elegans body wall muscle cells, using quantitative approaches that have been well established for in vitro amyloid-forming systems of the type favored by the reviewer. They calculate a reaction order of just 1.6, slightly higher than what would be expected for a monomeric nucleus but nevertheless fully consistent with our own conclusions when one accounts for the following two aspects of their approach. First, the polyQ tract in their construct is flanked by short poly-Histidine tracts on both sides. These charges very likely disfavor monomeric nucleation because all possible configurations of a four-stranded bundle position the beginning and end of the Q tract in close proximity, and Q40 is only just long enough to achieve monomeric nucleation in the absence of such destabilization. Second, the protein is fused to YFP, a weak homodimer (Landgraf et al., 2012; Snapp et al., 2003). With these two considerations, our model -- which was generated from polyQ tracts lacking flanking charges or an oligomeric fusion -- predicts that amyloid nucleation by their construct will occur more frequently as a dimer than a monomer. Indeed, their observed reaction order of 1.6 supports a predominantly dimeric nucleus. Like us and others, Sinnige et al. did not observe phase separation prior to amyloid formation. This is important because it not only argues against nucleation occurring in a condensate, it also suggests that the reaction order they calculated has not been limited by the concentration-buffering effect of phase separation.
While we agree that our conclusions rest heavily on DAmFRET data (for good reason), we do provide supporting evidence from molecular dynamics simulations, SDD-AGE, and microscopy.
To summarize, given the extreme limitations of in vitro experiments in this field, the breadth of our current study, and supporting findings from another lab using rigorous quantitative approaches, we feel that our claims are justified without in vitro data.
Rebuttal to the perceived incompatibility of monomeric nucleation with the existence of a critical concentration for amyloid
We appreciate that the concept of a monomeric nucleus can superficially appear inconsistent with the fact that crystalline solids such as polyQ amyloid have a saturating concentration, but this is only true if one neglects that polyQ amyloids are polymer crystals with intramolecular ordering. The perceived discrepancy is perhaps most easily dispelled by protein crystallography. Folded proteins form crystals. These crystals have critical concentrations, and the protein subunits within them each have intramolecular crystalline order (in the form of secondary structure). To extrapolate these familiar examples to our present finding with polyQ, one need only appreciate the now well-established phenomenon of secondary nucleation, whereby transient interactions of soluble species with the ordered species leads to their own ordering (Törnquist et al., 2018). Transience is important here because it implies that intramolecular ordering can in principle propagate even in solutions that are subsaturated with respect to bulk crystallization. This is possible in the present case because the pairing of sufficiently short beta strands (equivalent to “stems” in the polymer crystal literature) will be more stable intramolecularly than intermolecularly, due to the reduced entropic penalty of the former. Our elucidation that Q zipper ordering can occur with shorter strands intramolecularly than intermolecularly (Fig. S4C-D) demonstrates this fact. It is also evident from published descriptions of single molecule “crystals” formed in sufficiently dilute solutions of sufficiently long polymers (Hong et al., 2015; Keller, 1957; Lauritzen and Hoffman, 1960).
In suggesting that a saturating concentration for amyloid rules out monomeric nucleation, the reviewer assumes that the Q zipper-containing monomer must be stable relative to the disordered ensemble. This is not inherent to our claim and in fact opposes the definition of a nucleus. The monomeric nucleating structure need not be more stable than the disordered state, and monomers may very well be disordered at equilibrium at low concentrations. To be clear, our claim requires that the Q zipper-containing monomer is both on pathway to amyloid and less stable than all subsequent species that are on pathway to amyloid. The former requirement is supported by our extensive mutational analysis. The latter requirement is supported by our atomistic simulations showing the Q zipper-containing monomer is stabilized by dimerization (see our 2021 preprint). Hence, requisite ordering in the nucleating monomer is stabilized by intermolecular interactions. We provide in Author response image 1 an illustration to clarify what we believe to be the discrepancy between our claim and the reviewer’s interpretation.
Author response image 1.
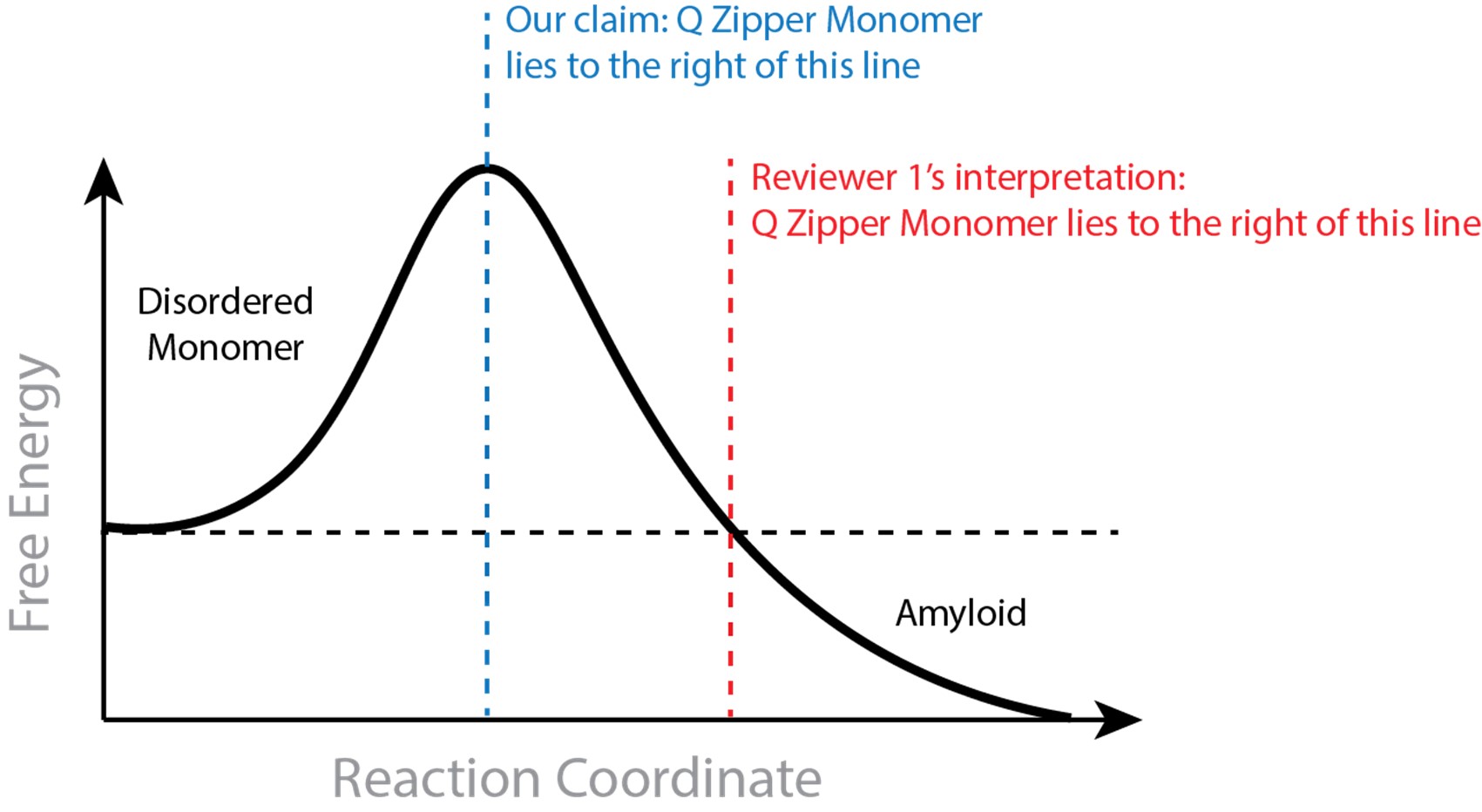
That the rate-limiting fluctuation for a crystalline phase can occur in a monomer can also be understood as a consequence of Ostwald’s rule of stages, which describes the general tendency of supersaturated solutes, including amyloid forming proteins (Chakraborty et al., 2023), to populate metastable phases en route to more stable phases (De Yoreo, 2022; Schmelzer and Abyzov, 2017). Our findings with polyQ are consistent with a general mechanism for Ostwald’s rule wherein the relative stabilities of competing polymorphs differ with the number of subunits (De Yoreo, 2022; Navrotsky, 2004). As illustrated in Fig. 6 of Navrotsky, a polymorph that is relatively stable at small particle sizes tends to give way to a polymorph that -- while initially unstable -- becomes more stable as the particles grow. The former is analogous to our early stage Q zipper composed of two short sheets with an intramolecular interface, while the latter is analogous to the later stage Q zipper composed of longer sheets with an intermolecular interface. Subunit addition stabilizes the latter more than the former, hence the initial Q zipper that is stabilized more by intra- than intermolecular interactions will mature with growth to one that is stabilized more by intermolecular interactions.
We apologize to the Pappu group for neglecting to cite Crick et al. 2013 in the current preprint. Contrary to the reviewer’s assessment, however, we find that the conclusions of this valuable study do more to support than to refute our findings. Briefly, Crick et al. investigated the aggregation of synthetic Q30 and Q40 peptides in vitro, wherein fibrils assembled from high concentrations of peptide were demonstrated to have saturating concentrations in the low micromolar range. As explained above, this finding of a saturating concentration does not refute our results. More relevant to the present work are their findings that “oligomers” accumulated over an hours-long timespan in solutions that are subsaturated with respect to fibrils, and these oligomers themselves have (nanomolar) critical concentrations. The authors postulated that the oligomers result from liquid–liquid demixing of intrinsically disordered polyglutamine. However, phase separation by a peptide is expected to fix its concentration in both the solute and condensed phases, and, because disordered phase separation is inherently faster than amyloid formation, the postulated explanation removes the driving force for any amyloid phase with a critical solubility greater than that of the oligomers. In place of this interpretation that truly does appear to -- in the reviewer’s words -- “contradict basic physical principles of how homopolymers self-assemble”, we interpret these oligomers as evidence of our Q zipper-containing self-poisoned multimers, rounded as an inherent consequence of self-poisoning (Ungar et al., 2005), and likely akin to semicrystalline spherulites that have been observed in other polymer crystal and amyloid-forming systems (Crist and Schultz, 2016; Vetri and Foderà, 2015). That Crick et al. also observed the formation of a relatively labile amyloid phase when the reactions were started with 50 uM peptide is unsurprising in light of the aforementioned kinetic advantage that large reaction volumes can confer to labile polymorphs, and that high concentrations (in this case, orders of magnitude higher than the likely physiological concentration of polyQ (Wild et al., 2015)) can favor the formation of labile amyloid polymorphs (Ohhashi et al., 2010). Indeed, a contemporaneous study by the Wetzel group using very similar peptide constructs and polyQ lengths -- but beginning with lower concentrations -- found that the relevant saturating concentrations for amyloid lie below their limit of detection of 100 nM (Sahoo et al., 2014).
Rebuttals to other critiques
The reviewer states that we found nucleation potential to require 60 Qs in a row. Our data are collectively consistent with nucleation occurring at and above approximately 36 Qs, a point repeated in the paper. The reviewer may be referring to our statement, ”Sixty residues proved to be the optimum length to observe both the pre- and post-nucleated states of polyQ in single experiments”. The purpose of this statement is simply to describe the practical consideration that led us to use 60 Qs for the bulk of our assays. We do appreciate that the fraction of AmFRET-positive cells is very low for lengths just above the threshold, especially Q40. They are nevertheless highly significant (p = 0.004 in [PIN+] cells, one-tailed T-test), and we will modify the figure and text to clarify this.
The reviewer characterizes self-poisoning as the hallmark of crystallization from polymer melts, which would be problematic for our conclusions if self-poisoning were limited to this non-physiological context. In fact the term was first used to describe crystallization from solution (Organ et al., 1989), wherein the phenomenon is more pronounced (Ungar et al., 2005).
Reviewer #2 (Public Review):
Numerous neurodegenerative diseases are thought to be driven by the aggregation of proteins into insoluble filaments known as "amyloids". Despite decades of research, the mechanism by which proteins convert from the soluble to insoluble state is poorly understood. In particular, the initial nucleation step is has proven especially elusive to both experiments and simulation. This is because the critical nucleus is thermodynamically unstable, and therefore, occurs too infrequently to directly observe. Furthermore, after nucleation much faster processes like growth and secondary nucleation dominate the kinetics, which makes it difficult to isolate the effects of the initial nucleation event. In this work Kandola et al. attempt to surmount these obstacles using individual yeast cells as microscopic reaction vessels. The large number of cells, and their small size, provides the statistics to separate the cells into pre- and post-nucleation populations, allowing them to obtain nucleation rates under physiological conditions. By systematically introducing mutations into the amyloid-forming polyglutamine core of huntingtin protein, they deduce the probable structure of the amyloid nucleus. This work shows that, despite the complexity of the cellular environment, the seemingly random effects of mutations can be understood with a relatively simple physical model. Furthermore, their model shows how amyloid nucleation and growth differ in significant ways, which provides testable hypotheses for probing how different steps in the aggregation pathway may lead to neurotoxicity.
In this study Kandola et al. probe the nucleation barrier by observing a bimodal distribution of cells that contain aggregates; the cells containing aggregates have had a stochastic fluctuation allowing the proteins to surmount the barrier, while those without aggregates have yet to have a fluctuation of suitable size. The authors confirm this interpretation with the selective manipulation of the PIN gene, which provides an amyloid template that allows the system to skip the nucleation event.
In simple systems lacking internal degrees of freedom (i.e., colloids or rigid molecules) the nucleation barrier comes from a significant entropic cost that comes from bringing molecules together. In large aggregates this entropic cost is balanced by attractive interactions between the particles, but small clusters are unable to form the extensive network of stabilizing contacts present in the larger aggregates. Therefore, the initial steps in nucleation incur an entropic cost without compensating attractive interactions (this imbalance can be described as a surface tension). When internal degrees of freedom are present, such as the conformational states of a polypeptide chain, there is an additional contribution to the barrier coming from the loss of conformational entropy required to the adopt aggregation-prone state(s). In such systems the clustering and conformational processes do not necessarily coincide, and a major challenge studying nucleation is to separate out these two contributions to the free energy barrier. Surprisingly, Kandola et al. find that the critical nucleus occurs within a single molecule. This means that the largest contribution to the barrier comes from the conformational entropy cost of adopting the beta-sheet state. Once this state is attained, additional molecules can be recruited with a much lower free energy barrier.
There are several caveats that come with this result. First, the height of the nucleation barrier(s) comes from the relative strength of the entropic costs compared to the binding affinities. This balance determines how large a nascent nucleus must grow before it can form interactions comparable to a mature aggregate. In amyloid nuclei the first three beta strands form immature contacts consisting of either side chain or backbone contacts, whereas the fourth strand is the first that is able to form both kinds of contacts (as in a mature fibril). This study used relatively long polypeptides of 60 amino acids. This is greater than the 20-40 amino acids found in amyloid-forming molecules like ABeta or IAPP. As a result, Kandola et al.'s molecules are able to fold enough times to create four beta strands and generate mature contacts intramolecularly. The authors make the plausible claim that these intramolecular folds explain the well-known length threshold (L~35) observed in polyQ diseases. The intramolecular folds reduce the importance of clustering multiple molecules together and increase the importance of the conformational states. Similarly, manipulating the sequence or molecular concentrations will be expected to manipulate the relative magnitude of the binding affinities and the clustering entropy, which will shift the relative heights of the entropic barriers.
The reviewer correctly notes that the majority of our manipulations were conducted with 60-residue long tracts (which corresponds to disease onset in early adulthood), and this length facilitates intramolecular nucleation. However, we also analyzed a length series of polyQ spanning the pathological threshold, as well as a synthetic sequence designed explicitly to test the model nucleus structure with a tract shorter than the pathological threshold, and both experiments corroborate our findings.
The authors make an important point that the structure of the nucleus does not necessarily resemble that of the mature fibril. They find that the critical nucleus has a serpentine structure that is required by the need to form four beta strands to get the first mature contacts. However, this structure comes at a cost because residues in the hairpins cannot form strong backbone or zipper interactions. Mature fibrils offer a beta sheet template that allows incoming molecules to form mature contacts immediately. Thus, it is expected that the role of the serpentine nucleus is to template a more extended beta sheet structure that is found in mature fibrils.
A second caveat of this work is the striking homogeneity of the nucleus structure they describe. This homogeneity is likely to be somewhat illusory. Homopolymers, like polyglutamine, have a discrete translational symmetry, which implies that the hairpins needed to form multiple beta sheets can occur at many places along the sequence. The asparagine residues introduced by the authors place limitations on where the hairpins can occur, and should be expected to increase structural homogeneity. Furthermore, the authors demonstrate that polyglutamine chains close to the minimum length of ~35 will have strict limitations on where the folds must occur in order to attain the required four beta strands.
We are unsure how to interpret the above statements as a caveat. We agree that increasing sequence complexity will tend to increase homogeneity, but this is exactly the motivation of our approach. We explicitly set out to determine the minimal complexity sequence sufficient to specify the nucleating conformation, which we ultimately identified in terms of secondary and tertiary structure. We do not specify which parts of a long polyQ tract correspond to which parts of the structure, because, as the reviewer points out, they can occur at many places. Hence, depending on the length of the polyQ tract, the nucleus we describe may have any length of sequence connecting the strand elements. We do not think that the effects of N-residue placement can be interpreted as a confounding influence on hairpin position because the striking even-odd pattern we observe implicates the sides of beta strands rather than the lengths. Moreover, we observe this pattern regardless of the residue used (Gly, Ser, Ala, and His in addition to Asn).
A novel result of this work is the observation of multiple concentration regimes in the nucleation rate. Specifically, they report a plateau-like regime at intermediate regimes in which the nucleation rate is insensitive to protein concentration. The authors attribute this effect to the "self-poisoning" phenomenon observed in growth of some crystals. This is a valid comparison because the homogeneity observed in NMR and crystallography structures of mature fibrils resemble a one-dimensional crystal. Furthermore, the typical elongation rate of amyloid fibrils (on the order of one molecule per second) is many orders of magnitude slower than the molecular collision rate (by factors of 10^6 or more), implying that the search for the beta-sheet state is very slow. This slow conformational search implies the presence of deep kinetic traps that would be prone to poisoning phenomena. However, the observation of poisoning in nucleation during nucleation is striking, particularly in consideration of the expected disorder and concentration sensitivity of the nucleus. Kandola et al.'s structural model of an ordered, intramolecular nucleus explains why the internal states responsible for poisoning are relevant in nucleation.
We thank the reviewer for noting the novelty and plausibility of the self-poisoning connection. We would like to elaborate on our finding that self-poisoning inhibits nucleation (in addition to elongation), as this could prove confusing to some readers. While self-poisoning is claimed to inhibit primary nucleation in the polymer crystal literature (Ungar et al., 2005; Zhang et al., 2018), the semantics of “nucleation” in this context warrants clarification. Technically, the same structure can be considered a nucleus in one context but not in another. The Q zipper monomer, even if it is rate-limiting for amyloid formation at low concentrations (and is therefore the “nucleus”), is not necessarily rate-limiting when self-poisoned at high concentrations. Whether it comprises the nucleus in this case depends on the rates of Q zipper formation relative to subunit addition to the poisoned state. If the latter happens slower than Q zipper formation de novo, it can be said that self-poisoning inhibits nucleation, regardless of whether the Q zipper formed. We suspect this to be the mechanism by which preemptive oligomerization blocks nucleation in the case of polyQ, though other mechanisms may be possible.
To achieve these results the authors used a novel approach involving a systematic series of simple sequences. This is significant because, while individual experiments showed seemingly random behavior, the randomness resolved into clear trends with the systematic approach. These trends provided clues to build a model and guide further experiments.
Reviewer #3 (Public Review):
Kandola et al. explore the important and difficult question regarding the initiating event that triggers (nucleates) amyloid fibril growth in glutamine-rich domains. The researchers use a fluorescence technique that they developed, dAMFRET, in a yeast system where they can manipulate the expression level over several orders of magnitude, and they can control the length of the polyglutamine domain as well as the insertion of interfering non-glutamine residues. Using flow cytometry, they can interrogate each of these yeast 'reactors' to test for self-assembly, as detected by FRET.
In the introduction, the authors provide a fairly thorough yet succinct review of the relevant literature into the mechanisms of polyglutamine-mediated aggregation over the last two decades. The presentation as well as the illustrations in Figure 1A and 1B are difficult to understand, and unfortunately, there is no clear description of the experimental technique that would allow the reader to connect the hypothetical illustrations to the measurement outcomes. The authors do not explain what the FRET signal specifically indicates or what its intensity is correlated to. FRET measures distance between donor and acceptor, but can it be reliably taken as an indicator of a specific beta-sheet conformation and of amyloid? Does the signal increase with both nucleation and with elongation, and is the signal intensity the same if, e.g., there were 5 aggregates of 10 monomers each versus 50 monomeric nuclei? Is there a reason why the AmFRET signal intensity decreases at longer Q even though the number of cells with positive signal increases? Does the number of positive cells increase with time? The authors state later that 'non-amyloid containing cells lacked AmFRET altogether', but this seems to be a tautology - isn't the lack of AmFRET taken as a proof of lack of amyloid? Overall, a clearer description of the experimental method and what is actually measured (and validation of the quantitative interpretation of the FRET signal) would greatly assist the reader in understanding and interpreting the data.
We believe the difficulty in understanding the illustrations in Figure 1A and 1B is inherent to the subject. We agree that elaborating how DAmFRET works would help the reader, and will add a few sentences to this end. Beyond this, we refer the reviewer and readers to our cited prior work describing the theory and interpretation of DAmFRET. Note that the y-axes of DAmFRET plots are not raw FRET but rather “AmFRET”, a ratio of FRET to total expression level. As explained thoroughly in our cited prior work, the discontinuity of AmFRET with expression level indicates that the high AmFRET-population formed via a disorder-to-order transition. When the query protein is predicted to be intrinsically disordered, the discontinuous transition to high AmFRET invariably (among hundreds of proteins tested in prior published and unpublished work) signifies amyloid formation as corroborated by SDD-AGE and tinctorial assays.
When performed using standard flow cytometry as in the present study, every AmFRET measurement corresponds to a cell-wide average, and hence does not directly inform on the distribution of the protein between different stoichiometric species. As there is only one fluorophore per protein molecule, monomeric nuclei have no signal. DAmFRET can distinguish cells expressing monomers from stable dimers from higher order oligomers (see e.g. Venkatesan et al. 2019), and we are therefore quite confident that AmFRET values of zero correspond to cells in which a vast majority of the respective protein is not in homo-oligomeric species (i.e. is monomeric or in hetero-complexes with endogenous proteins). The exact value of AmFRET, even for species with the same stoichiometry, will depend both on the effect of their respective geometries on the proximity of mEos3.1 fluorophores, and on the fraction of protein molecules in the species. Hence, we only attempt to interpret the plateau values of AmFRET (where the fraction of protein in an assembled state approaches unity) as directly informing on structure, as we did in Fig. S3A.
We believe that AmFRET decreases with longer polyQ because the mass fraction of fluorophore decreases in the aggregate, simply because the extra polypeptide takes up volume in the aggregate.
Yes, the fraction of positive cells in a discontinuous DAmFRET plot does increase with time. However, given the more laborious data collection and derivation of nucleation kinetics in a system with ongoing translation, especially across hundreds of experiments with other variables, ours is a snapshot measurement to approximately derive the relative contributions of intra- and intermolecular fluctuations to the nucleation barrier, rather than the barrier’s magnitude.
We will revise the tautological statement by removing “non-amyloid containing”.
The authors demonstrate that their assay shows that the fraction of cells with AmFRET signal increases strongly with an increase in polyQ length, with a 'threshold around 50-60 glutamines. This roughly correlates with the Q-length dependence of disease. The experiments in which asparagine or other amino acids are inserted at variable positions in the glutamine repeat are creative and thorough, and the data along with the simulations provide compelling support for the proposed Q zipper model. The experiments shown in Figure 5 are strongly supportive of a model where formation of the beta-sheet nucleus is within a monomer. This is a potentially important result, as there are conflicting data in the literature as to whether the nucleus in polyQ is monomer.
We thank the reviewer for these comments. We wish to clarify one important point, however, concerning the correlation of our data with the pathological length threshold. As we state in the first results section, “Our data recapitulated the pathologic threshold -- Q lengths 35 and shorter lacked AmFRET, indicating a failure to aggregate or even appreciably oligomerize, while Q lengths 40 and longer did acquire AmFRET in a length and concentration-dependent manner”. Hence, most of our experiments were conducted with 60Q not because it resembles the pathological threshold, but rather because it was most convenient for DAmFRET experiments.
I did not find the argument, that their data shows the Q zipper grows in two dimensions, compelling; there are more direct experimental methods to answer this question. I was also confused by the section that Q zippers poison themselves. It would be easier for the reader to follow if the authors first presented their results without interpretation. The data seem more consistent with an argument that, at high concentrations, non-structured polyQ oligomers form which interfere with elongation into structured amyloid assemblies - but such oligomers would not be zippers.
Self-poisoning is a widely observed and heavily studied phenomenon in polymer crystal physics, though it seems not yet to have entered the lexicon of amyloid biologists. We were new to this concept before it emerged as an extremely parsimonious explanation for our results. As described in the text, two pieces of evidence exclude the alternative mechanism suggested by the reviewer -- that non-structured oligomers form and subsequently engage and inhibit the template. Specifically, 1) inhibition occurs without any detectable FRET, even at high total protein concentration, indicating the species do not form in a concentration-dependent manner that would be expected of disordered oligomers; and 2) inhibition itself has strict sequence requirements that match those of Q zippers. Hence our data collectively suggest that inhibition is a consequence of the deposition of partially ordered molecules onto the templating surface.
Although some speculation or hypothesizing is perfectly appropriate in the discussion, overall the authors stretch this beyond what can be supported by the results. A couple of examples: The conclusion that toxicity arises from 'self-poisoned polymer crystals' is not warranted, as there is no relevant data presented in this manuscript. The authors refer to findings 'that kinetically arrested aggregates emerge from the same nucleating event responsible for amyloid formation', but I cannot recall any evidence for this statement in the results section.
We restricted any mention of toxicity to the introduction and a section in the discussion that is not worded as conclusive. Nevertheless, we will soften the subheading and text of the relevant section in the discussion to more clearly indicate the speculative nature of the statements.
We stand by our statement 'that kinetically arrested aggregates emerge from the same nucleating event responsible for amyloid formation', as this follows directly from self-poisoning.
Bibliography
Arseni D, Hasegawa M, Murzin AG, Kametani F, Arai M, Yoshida M, Ryskeldi-Falcon B. 2022. Structure of pathological TDP-43 filaments from ALS with FTLD. Nature 601:139–143. doi:10.1038/s41586-021-04199-3
Bansal A, Schmidt M, Rennegarbe M, Haupt C, Liberta F, Stecher S, Puscalau-Girtu I, Biedermann A, Fändrich M. 2021. AA amyloid fibrils from diseased tissue are structurally different from in vitro formed SAA fibrils. Nat Commun 12:1013. doi:10.1038/s41467-021-21129-z
Buell AK. 2017. The Nucleation of Protein Aggregates - From Crystals to Amyloid Fibrils. Int Rev Cell Mol Biol 329:187–226. doi:10.1016/bs.ircmb.2016.08.014
Chakraborty D, Straub JE, Thirumalai D. 2023. Energy landscapes of Aβ monomers are sculpted in accordance with Ostwald’s rule of stages. Sci Adv 9:eadd6921. doi:10.1126/sciadv.add6921
Crist B, Schultz JM. 2016. Polymer spherulites: A critical review. Prog Polym Sci 56:1–63. doi:10.1016/j.progpolymsci.2015.11.006
De Yoreo JJ. 2022. Casting a bright light on Ostwald’s rule of stages. Proc Natl Acad Sci USA 119. doi:10.1073/pnas.2121661119
Hong Y, Yuan S, Li Z, Ke Y, Nozaki K, Miyoshi T. 2015. Three-Dimensional Conformation of Folded Polymers in Single Crystals. Phys Rev Lett 115:168301. doi:10.1103/PhysRevLett.115.168301
Keller A. 1957. A note on single crystals in polymers: Evidence for a folded chain configuration. Philosophical Magazine 2:1171–1175. doi:10.1080/14786435708242746
Landgraf D, Okumus B, Chien P, Baker TA, Paulsson J. 2012. Segregation of molecules at cell division reveals native protein localization. Nat Methods 9:480–482. doi:10.1038/nmeth.1955
Lauritzen JI, Hoffman JD. 1960. Theory of Formation of Polymer Crystals with Folded Chains in Dilute Solution. J Res Natl Bur Stand A Phys Chem 64A:73–102. doi:10.6028/jres.064A.007
Navrotsky A. 2004. Energetic clues to pathways to biomineralization: precursors, clusters, and nanoparticles. Proc Natl Acad Sci USA 101:12096–12101. doi:10.1073/pnas.0404778101
Ohhashi Y, Ito K, Toyama BH, Weissman JS, Tanaka M. 2010. Differences in prion strain conformations result from non-native interactions in a nucleus. Nat Chem Biol 6:225–230. doi:10.1038/nchembio.306
Organ SJ, Ungar G, Keller A. 1989. Rate minimum in solution crystallization of long paraffins. Macromolecules 22:1995–2000. doi:10.1021/ma00194a078
Radamaker L, Baur J, Huhn S, Haupt C, Hegenbart U, Schönland S, Bansal A, Schmidt M, Fändrich M. 2021. Cryo-EM reveals structural breaks in a patient-derived amyloid fibril from systemic AL amyloidosis. Nat Commun 12:875. doi:10.1038/s41467-021-21126-2
Sahoo B, Singer D, Kodali R, Zuchner T, Wetzel R. 2014. Aggregation behavior of chemically synthesized, full-length huntingtin exon1. Biochemistry 53:3897–3907. doi:10.1021/bi500300c
Schmelzer JWP, Abyzov AS. 2017. How do crystals nucleate and grow: ostwald’s rule of stages and beyond In: Šesták J, Hubík P, Mareš JJ, editors. Thermal Physics and Thermal Analysis, Hot Topics in Thermal Analysis and Calorimetry. Cham: Springer International Publishing. pp. 195–211. doi:10.1007/978-3-319-45899-1_9
Schmidt M, Wiese S, Adak V, Engler J, Agarwal S, Fritz G, Westermark P, Zacharias M, Fändrich M. 2019. Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis. Nat Commun 10:5008. doi:10.1038/s41467-019-13038-z
Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B, Matsubara T, Tomita T, Ando T, Hasegawa K, Murayama S, Yoshida M, Hasegawa M, Scheres SHW, Goedert M. 2020. Structures of α-synuclein filaments from multiple system atrophy. Nature 585:464–469. doi:10.1038/s41586-020-2317-6
Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, Borgese N, Lippincott-Schwartz J. 2003. Formation of stacked ER cisternae by low affinity protein interactions. J Cell Biol 163:257–269. doi:10.1083/jcb.200306020
Törnquist M, Michaels TCT, Sanagavarapu K, Yang X, Meisl G, Cohen SIA, Knowles TPJ, Linse S. 2018. Secondary nucleation in amyloid formation. Chem Commun 54:8667–8684. doi:10.1039/c8cc02204f
Ungar G, Putra EGR, de Silva DSM, Shcherbina MA, Waddon AJ. 2005. The Effect of Self-Poisoning on Crystal Morphology and Growth Rates In: Allegra G, editor. Interphases and Mesophases in Polymer Crystallization I, Advances in Polymer Science. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 45–87. doi:10.1007/b107232
Vetri V, Foderà V. 2015. The route to protein aggregate superstructures: Particulates and amyloid-like spherulites. FEBS Lett 589:2448–2463. doi:10.1016/j.febslet.2015.07.006
Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JRC, Zetterberg H, Leavitt BR, Kuhn R, Tabrizi SJ, Macdonald D, Weiss A. 2015. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. The Journal of Clinical Investigation.
Yang Y, Arseni D, Zhang W, Huang M, Lövestam S, Schweighauser M, Kotecha A, Murzin AG, Peak-Chew SY, Macdonald J, Lavenir I, Garringer HJ, Gelpi E, Newell KL, Kovacs GG, Vidal R, Ghetti B, Ryskeldi-Falcon B, Scheres SHW, Goedert M. 2022. Cryo-EM structures of amyloid-β 42 filaments from human brains. Science 375:167–172. doi:10.1126/science.abm7285
Zhang X, Zhang W, Wagener KB, Boz E, Alamo RG. 2018. Effect of Self-Poisoning on Crystallization Kinetics of Dimorphic Precision Polyethylenes with Bromine. Macromolecules 51:1386–1397. doi:10.1021/acs.macromol.7b02745




