Author Response
The following is the authors’ response to the original reviews.
Reviewer #1:
Continuous attractor networks endowed with some sort of adaptation in the dynamics, whether that be through synaptic depression or firing rate adaptation, are fast becoming the leading candidate models to explain many aspects of hippocampal place cell dynamics, from hippocampal replay during immobility to theta sequences during run. Here, the authors show that a continuous attractor network endowed with spike frequency adaptation and subject to feedforward external inputs is able to account for several previously unaccounted aspects of theta sequences, including (1) sequences that move both forwards and backwards, (2) sequences that alternate between two arms of a T-maze, (3) speed modulation of place cell firing frequency, and (4) the persistence of phase information across hippocampal inactivations. I think the main result of the paper (findings (1) and (2)) are likely to be of interest to the hippocampal community, as well as to the wider community interested in mechanisms of neural sequences. In addition, the manuscript is generally well written, and the analytics are impressive. However, several issues should be addressed, which I outline below.
Major comments:
- In real data, population firing rate is strongly modulated by theta (i.e., cells collectively prefer a certain phase of theta - see review paper Buzsaki, 2002) and largely oscillates at theta frequency during run. With respect to this cyclical firing rate, theta sweeps resemble "Nike" check marks, with the sweep backwards preceding the sweep forwards within each cycle before the activity is quenched at the end of the cycle. I am concerned that (1) the summed population firing rate of the model does not oscillate at theta frequency, and (2) as the authors state, the oscillatory tracking state must begin with a forward sweep. With regards to (1), can the authors show theta phase spike preference plots for the population to see if they match data? With regards to (2), can the authors show what happens if the bump is made to sweep backwards first, as it appears to do within each cycle?
Thank you for raising these two important points. As the reviewer mentioned, experimental data does show that the population activity (e.g., calculated from the multiunit activity of tetrode recording) is strongly modulated by theta. While we mainly focused on sweeps of bump position, the populational activity also shows cyclical firing at the theta frequency (we added Fig. S7 to reflect this). This is also reflected in Fig. 4d where the bump height (representing the overall activity) oscillates at individual theta cycles. The underlying mechanism of cyclical population activity is as follows: the bump height is determined by the amount of input the neuron received (which located at the center of the bump). While the activity bump sweeps away from the external input, the center neuron receives less input from the external input, and hence the bump height is smaller. Therefore, not only the position sweeps around the external input, also the populational activity sweeps accordingly at the same frequency.
For the “Nike” check marks: we first clarify that the reason for we observed a forward sweep preceding a backward sweep is that we always force the artificial animal runs from left to right on the track where we treated “right” as “forward”. At the beginning of simulation, the external input to the network moves towards right, and therefore the activity bump starts from a position behind the animals and sweeps towards right (forward). In general, this means that the bump will never do a backward sweep first in our model. However, this does not mean that the forward sweeps precede the backward sweeps in each theta cycle. Experimentally, to determine the “0” phase of theta cycles, the LFP signal in CA1 was first bandpass filtered and then Hilbert transformed to get the phase at each time point. Then, a phase histogram of multiunit activity in CA1 was calculated across locomotor periods; the phase of maximal CA1 firing on the histogram was then defined to be “0” phase. Since we didn’t model LFP oscillation in the attractor model, we cannot obtain a “0” phase reference like the experimental procedure. Instead, we define the “0” phase using the “population activity quenched time”, where phase “0” is defined as the minimum population activity during oscillation cycles, which happens when the activity bump is farthest from the animal position. In this way, we observed a “Nike” pattern where the activity bump begins with a backward sweep towards the external input and then followed up with a forward sweep. This was showed in Fig. 3b in the main text.
- I could not find the width of the external input mentioned anywhere in the text or in the table of parameters. The implication is that it is unclear to me whether, during the oscillatory tracking state, the external input is large compared to the size of the bump, so that the bump lives within a window circumscribed by the external input and so bounces off the interior walls of the input during the oscillatory tracking phase, or whether the bump is continuously pulled back and forth by the external input, in which case it could be comparable to the size of the bump. My guess based on Fig 2c is that it is the latter. Please clarify and comment.
Thank you for your comment. We added the width of the external input to the text and table (see table 1). The bump is continuously pulled back and forth by the external input, as guessed by the reviewer. Experimentally, theta sweeps live roughly in the window of place field size. This is also true in our model, where theta sweep length depends on the strength of recurrent connections which determines the place field size. However, it also depends on the adaptation strength where large adaptation (more intrinsic mobility) leads to large sweep length. We presume that the reason for the reviewer had the guess that the bump may live within a window bounded by the external input is that we also set the width of external input comparable to the place field size (in fact, we don’t know how wide the external location input to the hippocampal circuits is in the biological brain, but it might be reasonable to set the external input width as comparable to the place field size, otherwise the location information conveyed to the hippocampus might be too dispersed). We added a plot in the SI (see Fig. S1) to show that when choosing a smaller external input width, but increasing the adaptation strength, the activity bump lives in a window exceeding the external input.
We clarified this point by adding the following text to line 159
“... It is noteworthy that the activity bump does not live within a window circumscribed by the external input bump (bouncing off the interior walls of the input during the oscillatory tracking state), but instead is continuously pulled back and forth by the external input (see Fig. S1)...”
- I would argue that the "constant cycling" of theta sweeps down the arms of a T-maze was roughly predicted by Romani & Tsodyks, 2015, Figure 7. While their cycling spans several theta cycles, it nonetheless alternates by a similar mechanism, in that adaptation (in this case synaptic depression) prevents the subsequent sweep of activity from taking the same arm as the previous sweep. I believe the authors should cite this model in this context and consider the fact that both synaptic depression and spike frequency adaptation are both possible mechanisms for this phenomenon. But I certainly give the authors credit for showing how this constant cycling can occur across individual theta cycles.
Thank you for raising this point. We added the citation of Romani & Tsodyks’ model in the context (line 304). As the reviewer pointed out, STD can also act as a potential mechanism for this phenomenon. We also gave the Romani & Tsodyks’ model credit for showing how this “cycling spanning several theta cycles” can account for the phenomenon of slow (~1Hz) and deliberative behaviors, namely, head scanning (Johson and Redish, 2007). We commented this in line 302
“... As the external input approaches the choice point, the network bump starts to sweep onto left and right arms alternatively in successive theta cycles (Fig. 5b and video 4; see also Romani and Tsodyks (2015) for a similar model of cyclical sweeps spanning several theta cycles) ...”
- The authors make an unsubstantiated claim in the paragraph beginning with line 413 that the Tsodyks and Romani (2015) model could not account for forwards and backwards sweeps. Both the firing rate adaptation and synaptic depression are symmetry breaking models that should in theory be able to push sweeps of activity in both directions, so it is far from obvious to me that both forward and backward sweeps are not possible in the Tsodyks and Romani model. The authors should either prove that this is the case (with theory or simulation) or excise this statement from the manuscript.
Thank you for your comment. Our claim about the Tsodyks and Romani (2015) model's inability to account for both forward and backward sweeps was inappropriate. We made this claim based on our own implementation of the Tsodyks and Romani (2015) model and didn’t find a parameter region where the bump oscillation shows both forward and backward sweeps. It might be due to the limited parameter range we searched from. Additionally, we also note some difference in these two models, where the Romani & Tsodyks’ model has an external theta input to the attractor network which prevent the bump to move further. This termination may also prevent the activity bump to move backward as well. We didn’t consider external theta input in our model, and the bump oscillation is based on internal dynamics. We have deleted that claim from line 424 in the revised paper, and revised that portion of the manuscript by adding the following text to line 424:
“…Different from these two models, our model considers firing rate adaptation to implement symmetry breaking and hence generates activity propagation. To prevent the activity bump from spreading away, their model considers an external theta input to reset the bump location at the end of each theta cycle, whereas our model generates an internal oscillatory state, where the activity bump travels back due to the attraction of external location input once it spreads too far away. Moreover, theoretical analysis of our model reveals how the adaptation strength affect the direction of theta sweeps, as well as offers a more detailed understanding of theta cycling in complex environments…”
- The section on the speed dependence of theta (starting with line 327) was very hard to understand. Can the authors show a more graphical explanation of the phenomenon? Perhaps a version of Fig 2f for slow and fast speeds, and point out that cells in the latter case fire with higher frequency than in the former?
Thank you for raising this valuable point. There are two different frequencies showed in Fig. 6 a,c &d. One is the bump oscillation frequency, the other is the firing frequency of single cell. To help understanding, we included experimental results (from Geisler et al, 2007) in Fig. 6a. It showed that when the animal increases its running speed, the LFP theta only increases a bit (compare the blue curve and the green curve), while the single cell firing rate oscillation frequency increases more. In our model, we first demonstrated this result using unimodal cells which have only significant phase precession (Fig. 6c). While the animal runs through the firing field of a place cell, the firing phase will always precess for half a cycle in total. Therefore, faster running speed means that the half cycle will be accomplished faster, and hence single cell oscillation frequency will be higher. We also predicted the results on bimodal cells (Fig. 6d). To make this point clearer, we modified Fig. 6 by including experimental results, and rewrote the paragraph as follows (line 337):
“…As we see from Fig. 3d and Fig. 4a&b, when the animal runs through the firing field of a place cell, its firing rate oscillates, since the activity bump sweeps around the firing field center of the cell. Therefore, the firing frequency of a place cell has a baseline theta frequency, which is the same as the bump oscillation frequency. Furthermore, due to phase precession, there will be a half cycle more than the baseline theta cycles as the animal runs over the firing field, and hence single cell oscillatory frequency will be higher than the baseline theta frequency (Fig. 6c). The faster the animal runs, the faster the extra half cycle is accomplished. Consequently, the firing frequency of single cells will increase more (a steeper slope in Fig. 6c red dots) than the baseline frequency.…”
- I had a hard time understanding how the Zugaro et al., (2005) hippocampal inactivation experiment was accounted for by the model. My intuition is that while the bump position is determined partially by the location of the external input, it is also determined by the immediate history of the bump dynamics as computed via the local dynamics within the hippocampus (recurrent dynamics and spike rate adaptation). So that if the hippocampus is inactivated for an arbitrary length of time, there is nothing to keep track of where the bump should be when the activity comes back online. Can the authors please explain more how the model accounts for this?
Thank you for the comments. The easiest way to understand how the model account for the experimental result from Zugaro et al., (2005) is from Eq. 8:
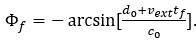
This equation says that the firing phase of a place cell is determined by the time the animal traveled through the place field, i.e., the location of the animal in the place field (with d0,c0 and vext all constant, and tf the only variable). No matter how long the hippocampus is inactivated (for an arbitrary length of time), once the external input is on, the new phase will continue from the new location of the animal in the place field. In other words, the peak firing phase keeps tracking the location of the animal. To make this point clearer, we modified Fig. 6 by including experimental results from Zugaro et al., (2005), and updated the description from line 356:
“…Based on the theoretical analysis (Eq. 8), we see that the firing phase is determined by the location of the animal in the place field, i.e., vext tf. This means that the firing phase keeps tracking the animal's physical location. No matter how long the network is inactivated, the new firing phase will only be determined by the new location of the animal in the place field. Therefore, the firing phase in the first bump oscillation cycle after the network perturbation is more advanced than the firing phase in the last bump oscillation cycle right before the perturbation, and the amount of precession is similar to that in the case without perturbation (Fig. 6e) …”
- Can the authors comment on why the sweep lengths oscillate in the bottom panel of Fig 5b during starting at time 0.5 seconds before crossing the choice point of the T-maze? Is this oscillation in sweep length another prediction of the model? If so, it should definitely be remarked upon and included in the discussion section.
We appreciate the reviewer’s valuable attention of this phenomenon. We thought it was a simulation artifact due to the parameter setting. However, we found that this phenomenon is quite robust to different parameter settings. While we haven’t found a theoretical explanation, we provide a qualitative explanation for it: this length oscillation frequency may be coupled with the time constant of the firing rate adaptation. Specifically, for a longer sweep, the neurons at the end of the sweep are adapted (inhibited), and hence the activity bump cannot travel that long in the next round. Therefore, the sweep length is shorter compared to the previous one. In the next round, the bump will sweep longer again because those neurons have recovered from the previous adaptation effect. We think this length oscillation is quite interesting and will check that in the experimental data in future works. We added this point in the main text as a prediction in line 321:
“…We also note that there is a cyclical effect in the sweep lengths across oscillation cycles before the animal enters the left or right arm (see Fig. 5b lower panel), which may be interesting to check in the experimental data in future work (see Discussion for more details) …”
And line 466:
“…Our model of the T-maze environment showed an expected phenomenon that as the animal runs towards the decision point, the theta sweep length also shows cyclical patterns (Fig. 5b lower panel). An intuitive explanation is that, due to the slow dynamics in firing rate adaptation (with a large time constant compared to neural firing), a long sweep leads to an adaptation effect on the neurons at the end of the sweep path. Consequently, the activity bump cannot travel as far due to the adaptation effect on those neurons, resulting in a shorter sweep length compared to the previous one. In the next round, the activity bump exhibits a longer sweep again because those neurons have recovered from the previous adaptation effect. We plan to test this phenomenon in future experiments...”
- Perhaps I missed this, but I'm curious whether the authors have considered what factors might modulate the adaptation strength. In particular, might rat speed modulate adaptation strength? If so, would have interesting predictions for theta sequences at low vs high speeds.
Thank you for raising up this important point. As we pointed out in line 279: “…the experimental data (Fernandez et al, 2017) has indicated that there is a laminar difference between unimodal cells and bimodal cells, with bimodal cells correlating more with the firing patterns of deep CA1 neurons and unimodal cells with the firing patterns of superficial CA1 neurons. Our model suggests that this difference may come from the different adaptation strengths in the two layers…”. Our guess is that the adaptation strength might reflect some physiological differences of place cells in difference pyramidal layers in the hippocampus. For example, place cells in superficial layer and deep layer receive different amount of input from MEC and sensory cortex, and such difference may contribute to a different effect of adaptation of the two populations of place cells.
Our intuition is that animal’s running speed may not directly modulate the adaptation strength. Note that the effect of adaptation and adaptation strength are different. As the animal rapidly runs across the firing field, the place cell experiences a dense firing (in time), therefore the adaptation effect is large; as the animal slowly runs across the field, the place cell experiences sparse firing (in time), and hence the adaptation effect is small. In these two situations, the adaption strength is fixed, but the difference is due to the spike intervals.
From Eq. 45-47, our theoretical analysis shows several predictions of theta sequences regarding to the parameters in the network. For example, how the sweep length varies when the running speed changes in the network. We simulated the network in both low running speed and high running speed (while kept all other parameters fixed), and found that the sweep length at low speed is larger than that at high speed. This is different from previously data, where they showed that the sweep length increases as the animal runs faster (Maurer et al, 2012). However, we are not sure how other parameters are changed in the biological brain as the animal runs faster, e.g., the external input strength and the place field width might also vary as confounds. We will explore this more in the future and investigate how the adaptation strength is modulated in the brain.
- I think the paper has a number of predictions that would be especially interesting to experimentalists but are sort of scattered throughout the manuscript. It would be beneficial to have them listed more prominently in a separate section in the discussion. This should include (1) a prediction that the bump height in the forward direction should be higher than in the backward direction, (2) predictions about bimodal and unimodal cells starting with line 366, (3) prediction of another possible kind of theta cycling, this time in the form of sweep length (see comment above), etc.
Thank you for pointing this out. We updated the manuscript by including a paragraph in Discussion summarizing the prediction we made throughout the manuscript (from line 459):
‘’…Our model has several predictions which can be tested in future experiments. For instance, the height of the activity bump in the forward sweep window is higher than that in the backward sweep window (Fig. 4c) due to the asymmetric suppression effect from the adaptation. For bimodal cells, they will have two peaks in their firing frequency as the animal runs across the firing fields, with one corresponding to phase precession and the other corresponding to phase procession. Similar to unimodal cells, both the phase precession and procession of a bimodal cell after transient intrahippocampal perturbation will continue from the new location of the animal (Fig. S5). Interestingly, our model of the T-maze environment showed an expected phenomenon that as the animal runs towards the decision point, the theta sweep length also shows cyclical patterns (Fig. 5b lower panel). An intuitive explanation is that, due to the slow dynamics in firing rate adaptation (with a large time constant compared to neural firing), a long sweep leads to an adaptation effect on the neurons at the end of the sweep path. Consequently, the activity bump cannot travel as far due to the adaptation effect on those neurons, resulting in a shorter sweep length compared to the previous one. In the next round, the activity bump exhibits a longer sweep again because those neurons have recovered from the previous adaptation effect. We plan to test this phenomenon in future experiments…’
Reviewer #2:
In this work, the authors elaborate on an analytically tractable, continuous-attractor model to study an idealized neural network with realistic spiking phase precession/procession. The key ingredient of this analysis is the inclusion of a mechanism for slow firing-rate adaptation in addition to the otherwise fast continuous-attractor dynamics. The latter which continuous-attractor dynamics classically arises from a combination of translation invariance and nonlinear rate normalization. For strong adaptation/weak external input, the network naturally exhibits an internally generated, travelling-wave dynamics along the attractor with some characteristic speed. For small adaptation/strong external stimulus, the network recovers the classical externally driven continuous-attractor dynamics. Crucially, when both adaptation and external input are moderate, there is a competition with the internally generated and externally generated mechanism leading to oscillatory tracking regime. In this tracking regime, the population firing profile oscillates around the neural field tracking the position of the stimulus. The authors demonstrate by a combination of analytical and computational arguments that oscillatory tracking corresponds to realistic phase precession/procession. In particular the authors can account for the emergence of a unimodal and bimodal cells, as well as some other experimental observations with respect the dependence of phase precession/procession on the animal's locomotion. The strengths of this work are at least three-fold: 1) Given its simplicity, the proposed model has a surprisingly large explanatory power of the various experimental observations. 2) The mechanism responsible for the emergence of precession/procession can be understood as a simple yet rather illuminating competition between internally driven and externally driven dynamical trends. 3) Amazingly, and under some adequate simplifying assumptions, a great deal of analysis can be treated exactly, which allows for a detailed understanding of all parametric dependencies. This exact treatment culminates with a full characterization of the phase space of the network dynamics, as well as the computation of various quantities of interest, including characteristic speeds and oscillating frequencies.
- As mentioned by the authors themselves, the main limitation of this work is that it deals with a very idealized model and it remains to see how the proposed dynamical behaviors would persist in more realistic models. For example, the model is based on a continuous attractor model that assumes perfect translation-invariance of the network connectivity pattern. Would the oscillating tracking behavior persist in the presence of connection heterogeneities?
Thank you for raising up this important point. Continuous attractor models have been widely used in modeling hippocampal neural circuits (see McNaughton et al, 2006 for a review), and researchers often assumed that there is a translation-invariance structure in these network models. The theta sweep state we presented in the current work is based on the property of the continuous attractor state. We do agree with the reviewer that the place cell circuit might not be a perfect continuous attractor network. For a simpler case where the connection weights are sampled from a Gaussian distribution around J_0, the theta sweep state still exhibit in the network (see Fig. S8 for an example). We also believe that the model can be extended to more complex cases where there exist over-representations of the “home” location and decision points in the real environment, i.e., the heterogeneity is not random, but has stronger connections near those locations, then the theta sweeps will be more biased to those location. However, if the heterogeneity breaks the continuous attractor state, the theta sweep state may not be presented in the network.
- Can the oscillating tracking behavior be observed in purely spiking models as opposed to rate models as considered in this work?
Thank you for pointing this out. The short answer is yes. If the translation-invariance of the network connectivity pattern hold in the network, i.e., the spiking network is still a continuous attractor network (see the work from Tsodyks et al, 1996; and from Yu et al. "Spiking continuous attractor neural networks with spike frequency adaptation for anticipative tracking"), then the adaptation, which has the mathematical form of spike frequency adaptation (instead of firing rate adaptation), will still generate sweep state of the activity bump. We here chose the rate-based model because it is analytically tractable, which gives us a better understanding of the underlying dynamics. Many of the continuous attractor model related to spatial tuning cell populations are rate-based (see examples Zhang 1996; Burak & Fiete 2009). However, extending to spike-based model would be straightforward.
- Another important limitation is that the system needs to be tuned to exhibit oscillation within the theta range and that this tuning involves a priori variable parameters such as the external input strength. Is the oscillating-tracking behavior overtly sensitive to input strength variations?
Thank you for pointing this out. In rodent studies, theta sequences are thought to result from the integration of both external inputs conveying sensory-motor information, and intrinsic network dynamics possibly related to memory processes (see Drieu and Zugaro 2019; Drieu at al, 2018). We clarified here that, in our modeling work, the generation of theta sweeps also depends on both the external input and the intrinsic dynamics (induced by the firing rate adaptation). Therefore, we don’t think the dependence of theta sweeps on the prior parameter – the external input strength – is a limitation here. We agreed with the reviewer that the system needs to be tuned to exhibit oscillation within the theta range. However, the parameter range of inducing oscillatory state is relatively large (see Fig. 2g in the main text). It will be interesting to investigate (and find experimental evidence) how the biological system adjusts the network configuration to implement the sweep state in network dynamics.
- The author mentioned that an external pacemaker can serve to drive oscillation within the desired theta band but there is no evidence presented supporting this.
Thank you for pointing this out. We made this argument based on our initial simulation before but didn’t go into the details of that. We have deleted that argument in the discussion and rewrote that part. We will carry out more simulations in the future to verify if this is true. See our changes from line 418 to line 431:
“... A representative model relying on neuronal recurrent interactions is the activation spreading model. This model produces phase precession via the propagation of neural activity along the movement direction, which relies on asymmetric synaptic connections. A later version of this model considers short-term synaptic plasticity (short-term depression) to implicitly implement asymmetric connections between place cells, and reproduces many other interesting phenomena, such as phase precession in different environments. Different from these two models, our model considers firing rate adaptation to implement symmetry breaking and hence generates activity propagation. To prevent the activity bump from spreading away, their model considers an external theta input to reset the bump location at the end of each theta cycle, whereas our model generates an internal oscillatory state, where the activity bump travels back due to the attraction of external location input once it spreads too far away. Moreover, theoretical analysis of our model reveals how the adaptation strength affect the direction of theta sweeps, as well as offers a more detailed understanding of theta cycling in complex environments...”
- A final and perhaps secondary limitation has to do with the choice of parameter, namely the time constant of neural firing which is chosen around 3ms. This seems rather short given that the fast time scale of rate models (excluding synaptic processes) is usually given by the membrane time constant, which is typically about 15ms. I suspect this latter point can easily be addressed.
Thank you for pointing this out. The time constant we currently chose is relatively short as used in other studies. We conducted additional simulation by adjusting the time constant to 10ms, and the results reported in this paper remain consistent. Please refer to Fig S9 for the results obtained with a time constant of 10 ms.
Reviewer #3:
With a soft-spoken, matter-of-fact attitude and almost unwittingly, this brilliant study chisels away one of the pillars of hippocampal neuroscience: the special role(s) ascribed to theta oscillations. These oscillations are salient during specific behaviors in rodents but are often taken to be part of the intimate endowment of the hippocampus across all mammalian species, and to be a fundamental ingredient of its computations. The gradual anticipation or precession of the spikes of a cell as it traverses its place field, relative to the theta phase, is seen as enabling the prediction of the future - the short-term future position of the animal at least, possibly the future in a wider cognitive sense as well, in particular with humans. The present study shows that, under suitable conditions, place cell population activity "sweeps" to encode future positions, and sometimes past ones as well, even in the absence of theta, as a result of the interplay between firing rate adaptation and precise place coding in the afferent inputs, which tracks the real position of the animal. The core strength of the paper is the clarity afforded by the simple, elegant model. It allows the derivation (in a certain limit) of an analytical formula for the frequency of the sweeps, as a function of the various model parameters, such as the time constants for neuronal integration and for firing rate adaptation. The sweep frequency turns out to be inversely proportional to their geometric average. The authors note that, if theta oscillations are added to the model, they can entrain the sweeps, which thus may superficially appear to have been generated by the oscillations.
- The main weakness of the study is the other side of the simplicity coin. In its simple and neat formulation, the model envisages stereotyped single unit behavior regulated by a few parameters, like the two time constants above, or the "adaptation strength", the "width of the field" or the "input strength", which are all assumed to be constant across cells. In reality, not only assigning homogeneous values to those parameters seems implausible, but also describing e.g. adaptation with the simple equation included in the model may be an oversimplification. Therefore, it remains important to understand to what extent the mechanism envisaged in the model is robust to variability in the parameters or to eg less carefully tuned afferent inputs.
Thank you for pointing out this important question. As the reviewer pointed out, there is an oversimplification in our model compared to the real hippocampal circuits (also see Q1 and Q3 from reviewer2). We also pointed out that in the main text line 504:
“…Nevertheless, it is important to note that the CANN we adopt in the current study is an idealized model for the place cell population, where many biological details are missed. For instance, we have assumed that neuronal synaptic connections are translation-invariant in the space...”
To investigate model robustness to parameter setting, we divided all the parameters into two groups. The first group of parameters determines the bump state, i.e., width of the field a, neuronal density ρ, global inhibition strength k, and connection strength J_0. The second group of parameters determines the bump sweep state (which based on the existence of the bump state), i.e., the input strength α and the adaptation strength m. For the first group of parameters, we refer the reviewer to the Method part: stability analysis of the bump state. This analysis tells us the condition when the continuous attractor state holds in the network (see Eq. 20, which guides us to perform parameter selection). For the second group of parameters, we refer the reviewer to Fig. 2g, which tells us when the bump sweep state occurs regarding to input strength and adaptation strength. When the input strength is small, the range of adaptation strength is also small (to get the bump sweep state). However, as the input strength increases, we can see from Fig. 2g that the range of adaptation strength (to get the bump sweep state) also linearly increases. Although there exists other two state in the network when the two parameters are set out of the colored area in Fig. 2g, the parameter range of getting sweep state is also large, especially when the input strength value is large, which is usually the case when the animal actively runs in the environment.
To demonstrate how the variability affect the results, we added variability to the connection weights by sampling the connection weights from a Gaussian distribution around J_0 (this introduces heterogeneity in the connection structure). We found that the bump sweep state still holds in this condition (see Fig. S8 as well as Q1 from reviewer2). For the variability in other parameter values, the results will be similar. Although adding variability to these parameters will not bring us difficulty in numerical simulation, it will make the theoretical analysis much more difficult.
- The weak adaptation regime, when firing rate adaptation effectively moves the position encoded by population activity slightly ahead of the animal, is not novel - I discussed it, among others, in trying to understand the significance of the CA3-CA1 differentiation (2004). What is novel here, as far as I know, is the strong adaptation regime, when the adaptation strength m is at least larger than the ratio of time constants. Then population activity literally runs away, ahead of the animal, and oscillations set in, independent of any oscillatory inputs. Can this really occur in physiological conditions? A careful comparison with available experimental measures would greatly strengthen the significance of this study.
Thank you for raising up this interesting question.
Re: “…firing rate adaptation effectively moves the position encoded by population activity slightly ahead of the animal, is not novel…”, We added Treves, A (2004) as a citation when we introduce the firing rate adaptation in line 116
To test if the case of “…the adaptation strength m is at least larger than the ratio of time constants…” could occur in physiological conditions, it requires a measure of the adaptation strength as well as the time constant of both neuron firing and adaptation effect. The most straightforward way would be in vivo patch clamp recording of hippocampal pyramidal neurons when the animal is navigating an environment. This will give us a direct measure of all these values. However, we don’t have these data to verify this hypothesis yet. Another possible way of measure these values is through a state-space model. Specifically, we can build a state space model (considering adaptation effect in spike release) by taking animal’s position as latent dynamics, and recorded spikes as observation, then infer the parameters such as adaptation strength and time constant in the slow dynamics. Previous work of state-space models (without firing rate adaptation) in analyzing theta sweeps and replay dynamics have been explored by Denovellis et al. (2021), as well as Krause and Drugowitsch (2022). We think it might be doable to infer the adaptation strength and adaptation time constant in a similar paradigm in future work. We thank the reviewer for pointing out that and hope our replies have clarified the concerns of the reviewer.