Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorMichael CzechUniversity of Massachusetts Chan Medical School, Worcester, United States of America
- Senior EditorBenoit KornmannUniversity of Oxford, Oxford, United Kingdom
Reviewer #1 (Public Review):
Previous reports suggested an association between ceramide accumulation in skeletal muscle and disruption of insulin signaling and metabolic dysregulation. Mechanistically, however, how intracellular ceramide attenuates insulin action and reduces metabolism is not fully understood. It was suggested that insulin receptor (IR) signaling to PI3-K/AKT is inhibited by elevated intracellular ceramide. However, other studies failed to demonstrate an inhibitory effect of ceramide on PI3K/AKT. More recently, a study was published describing that intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle (PMID: 29415895). In the present study, Diaz-Vegas and colleagues used an in vitro system to investigate this topic further and better understand how intracellular ceramide accumulation causes cellular insulin resistance and metabolic dysregulations in cultured myocytes.
The authors applied multiple methods to achieve this goal. Among these procedures are:
The overexpression of enzymes involved in mitochondrial ceramide synthesis and degradation;
Treatments of myocytes with different pharmacological tools to validate their findings;
Mitochondrial proteomics and lipidomics analyses.
The effects of these experimental conditions and treatment on intracellular lipids contents, mitochondrial functions, and insulin signaling in myocytes were then evaluated.
Findings:
The authors' findings indicate that incubation of myocytes with palmitate increases mitochondrial ceramide and reduces the insulin-stimulated GLUT4-HA translocation to the myocyte surface without affecting AKT activation. The elevation in mitochondrial ceramide lowers the coenzyme Q levels e depletes the electron transport chain (ETC) components, impairing mitochondrial respiration. Such mitochondrial dysfunction appears to attenuate the translocation of GLUT4-HA to the plasma membrane of the L6-myotubule. Also, mitochondrial proteomic analysis revealed an association of insulin sensitivity with mitochondrial ceramide and ETC expression levels in human muscle.
Based on these findings, the authors propose a mechanism whereby the building up of ceramide inside mitochondria depletes CoQ and compromise mitochondrial respiratory complexes, raising ROS. The resulting mitochondrial dysfunction causes insulin resistance in cultured myocytes. They postulate that CoQ depletion links ceramides with insulin resistance and define the respirasome as a critical connection between ceramides and mitochondrial dysfunction.
Relevance and critiques:
This original study provides direct evidence that mitochondrial ceramide accumulation depletes CoQ and downregulates multiple ETC components in myocytes. Consequently, elevation in the levels of reactive oxygen species (ROS) and mitochondrial dysfunctions occur. The authors proposed that such mitochondrial dysregulation attenuates insulin-stimulated GLUT4 translocation to the plasma membrane of L6-myotubules. Moreover, mitochondrial ceramide accumulation does not affect insulin action on AKT activation.
Overall, this is a well-done study, showing that in obesity, elevated mitochondrial ceramide suppresses mitochondrial function and attenuates insulin action on glucose transporter GLUT4 translocation into the myocyte surface. The main conclusion is supported by the results presented. The study also applied multiple methods and described several experiments designed to test the author's central hypothesis.
Importantly, these new findings shed light on possible cellular mechanisms whereby ectopic fat deposition in skeletal muscle drives insulin resistance and metabolism dysregulation. The results demonstrating that alterations in mitochondrial ceramide are sufficient to attenuate insulin-stimulated GLUT4 trafficking in cultured myocytes are very interesting. Well-done.
Comments for further discussion and suggestions:
Although the authors' results suggest that higher mitochondrial ceramide levels suppress cellular insulin sensitivity, they rely solely on a partial inhibition (i.e., 30%) of insulin-stimulated GLUT4-HA translocation in L6 myocytes. It would be critical to examine how much the increased mitochondrial ceramide would inhibit insulin-induced glucose uptake in myocytes using radiolabel deoxy-glucose.
Another important question to be addressed is whether glycogen synthesis is affected in myocytes under these experimental conditions. Results demonstrating reductions in insulin-stimulated glucose transport and glycogen synthesis in myocytes with dysfunctional mitochondria due to ceramide accumulation would further support the authors' claim.
In addition, it would be critical to assess whether the increased mitochondrial ceramide and consequent lowering of energy levels affect all exocytic pathways in L6 myoblasts or just the GLUT4 trafficking. Is the secretory pathway also disrupted under these conditions?
Reviewer #2 (Public Review):
The findings reported by Diaz-Vegas et al. extend those described in a previous paper from the same group establishing a role for mitochondrial CoQ depletion in the development of insulin resistance in muscle and adipose tissue (Fazakerley, 2018). In this new report, investigators sought to determine whether CoQ depletion contributes to insulin resistance caused by palmitate exposure and/or intracellular ceramide accumulation. To this end, researchers employed a widely used in vitro model of insulin resistance wherein L6 myocytes develop impaired Glut4 translocation upon exposure to palmitate (in this case, 150 uM for 16 hours). This model was combined with a variety of pharmacologic and genetic manipulations aimed at augmenting or inhibiting CoQ biosynthesis and/or ceramide biosynthesis, specifically in mitochondria. This series of experiments produced a valuable and provocative body of evidence positioning CoQ depletion downstream of mitochondrial ceramide accumulation and necessary for both palmitate- and ceramide-induced insulin resistance in L6 myocytes. Investigators concluded that mitochondrial ceramides, CoQ depletion and respiratory dysfunction are part of a core pathway leading to insulin resistance.
Strengths:
The study provides exciting, first-time evidence linking palmitate-induced insulin resistance to ceramide accumulation within the mitochondria and subsequent depletion of CoQ. Ceramide accumulation specifically in mitochondria was found to be necessary and sufficient to cause insulin resistance in cultured L6 myocytes.
The in vitro experiments featured a set of mitochondrial-targeted genetic manipulations that permitted up/down-regulation of ceramide levels specifically in the mitochondrial compartment. Genetically induced mitochondrial ceramide accumulation led to CoQ depletion, which was accompanied by increased ROS production and diminution of ETC proteins and OXPHOS capacity and impaired insulin action, thereby establishing cause/effect.
Analysis of mitochondria isolated from human muscle biopsies obtained from individuals with disparate metabolic phenotypes revealed a positive correlation between complex I proteins and insulin sensitivity and a negative correlation with mitochondrial ceramide content. While it is likely that many factors contribute to these correlations, the results support the possibility that the ceramide/CoQ mechanism might be relevant to glucose control in humans.
These important findings offer valuable new insights into mechanisms that connect ceramides to insulin resistance and mitochondrial dysfunction, and could inform new therapeutic approaches towards improved glucose control.
Weaknesses:
The mechanistic aspect of the work and conclusions put forth rely heavily on studies performed in cultured myocytes, which are highly glycolytic and generally viewed as a poor model for studying muscle metabolism and insulin action. Nonetheless, the findings provide a strong rationale for moving this line of investigation into mouse gain/loss of function models.
One caveat of the approach taken is that exposure of cells to palmitate alone is not reflective of in vivo physiology. It would be interesting to know if similar effects on CoQ are observed when cells are exposed to a more physiological mixture of fatty acids that includes a high ratio of palmitate, but better mimics in vivo nutrition.
While the utility of targeting SMPD5 to the mitochondria is appreciated, the results in Figure 5 suggest that this manoeuvre caused a rather severe form of mitochondrial dysfunction. This could be more representative of toxicity rather than pathophysiology. It would be helpful to know if these same effects are observed with other manipulations that lower CoQ to a similar degree. If not, the discrepancies should be discussed.
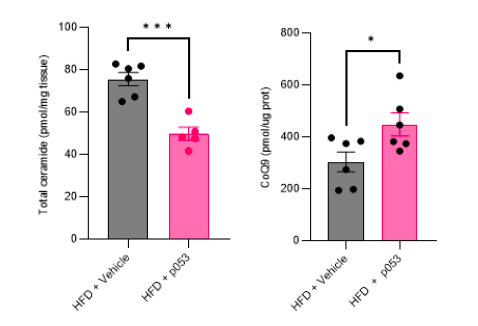
The conclusions could be strengthened by more extensive studies in mice to assess the interplay between mitochondrial ceramides, CoQ depletion and ETC/mitochondrial dysfunction in the context of a standard diet versus HF diet-induced insulin resistance. Does P053 affect mitochondrial ceramide, ETC protein abundance, mitochondrial function, and muscle insulin sensitivity in the predicted directions?