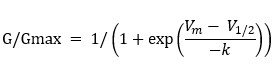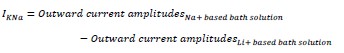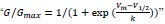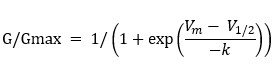Author Response
The following is the authors’ response to the original reviews.
We would like to thank the editor and all the reviewers for their time and thoughtful consideration of our manuscript. We appreciate the valuable comments. Our provisional response to the “public review” has been published and now we have corrected factual errors and enhanced the clarity of writings based on the “recommendations for the authors.” We believe these corrections will improve the quality and accuracy of our manuscript.
Specific responses to the reviewers' recommendations for the authors are as follows:
Reviewer #1 (Recommendations For The Authors):
- Is the Slack current amplitude dependent on the Nav subtype? Differences in Slack current amplitude might explain the sensitization of Slack to quinidine.
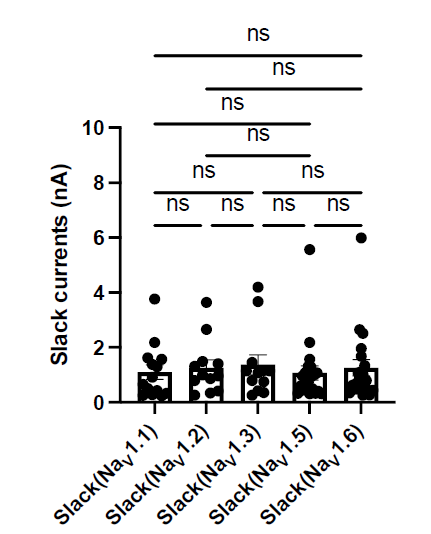
We appreciate the reviewer for raising this point. We examined Slack current amplitudes upon co-expression of Slack with specific NaV subtypes in HEK293 cells. The results have shown that there are no significant differences in Slack current amplitudes upon co-expression of Slack with different NaV channel subtypes (Author response image 1), suggesting whole-cell Slack current amplitudes cannot explain the varied ability of NaV subtypes to sensitize Slack to quinidine blockade.
Author response image 1.
The amplitudes of Slack currents upon co-expression of Slack with specific NaV subtypes in HEK293 cells. ns, p > 0.05, one-way ANOVA followed by Bonferroni’s post hoc test.
- Is the open probability changed by the presence of Nav1.6 and/or by the other Nav subtypes? Changes in open probability might explain the Nav1.6 induced sensitization of Slack to quinidine block.
We appreciate the reviewer for raising this point. To investigate the effect of different NaV channel subtypes on Slack open probability, we will perform the single-channel recordings in future studies.
- Could the authors elaborate more on the coupling between INaT mediated sensitization of Slack to block by quinidine and the Nav1.6 N-and C-tail induced sensitization?
We appreciate the reviewer for raising this point. We fully agree the importance of investigating the detailed mechanism underlying the sensitization of Slack to quinidine blockade. To address the questions, we plan to employ structural biological methods, such as cryo-electron microscopy (cryo-EM).
- Line 85: The authors use an outdated nomenclature of AMPAR subtypes. I would suggest changing to GluA1, GluA2, GluA3 and GluA4.
We appreciate the reviewer’s suggestion. We have changed the term “GluR” to “GluA” in the revised manuscript.
The authors do not explain the rationale by using the different homomeric AMPAR subtypes. Most often the AMPARs express as heteromeric receptors decorated by auxiliary subunits. Also, is the GluA2 the edited version?
We thank the reviewer for raising this point. While AMPARs are often expressed as heteromeric receptors with auxiliary subunits, we focused on the homomeric AMPAR subtypes for initial screening. Through our investigation, we found no significant effects on sensitizing Slack to quinidine blockade. Additionally, the GluA2 used in our study is unedited.
- Line 144: I expect a reduction in current amplitude caused by blocking INaT and INaP is tested at +100mV?
We thank the reviewer for raising this point. The reduction in current amplitude was indeed tested at +100 mV and we have included this information in the revised manuscript.
- Line 157 and line 162: Reference to Supplementary table S3 should be Table S2.
We thank the reviewer for pointing this out. The reference to "Table S3" has been corrected to "Table S2" in the revised manuscript.
- How many times did the authors repeat the co-immunoprecipitation? Some of the bands are very weak, and repeats are necessary for all blots.
We thank the reviewer for raising this concern. We performed the co-immunoprecipitation experiments three times independently.
- Line 288: The authors are showing the chimeric construct in Figures 7A and B but are referring to the full length Nav1.6 in the main text line 288.
We apologize for the confusion. We have clarified in the revised manuscript that we used
NaV1.5/6NC in our study.
- Figure 1 line 23: 1 uM quinidine must be 30 uM quinidine?
We thank the reviewer for catching this error. We have corrected the concentration value in the caption of Figure 1 from "1 μΜ" to "30 μΜ" in the revised manuscript.
- Figure 2 line 53: I expect IC50 is measured at +100mV? Same question for line 60 in same figure text.
We thank the reviewer for pointing this out. We have now included this information in the revised manuscript.
- Figure 4B color coding is confusing.
We apologize for the confusion. We would like to clarify that Fig. 4B illustrates the domain architecture of the human NaV channel pore-forming α subunit, and we have changed the color from dark blue to black in the revised figure.
- Figure S6: Text for figure S6E and S6F has been swapped (line 96 to 106).
We thank the reviewer for raising this point. We have rectified the swapped captions for
Fig. S6E and Fig. S6F in the revised manuscript.
- Methods section line 652: Kainite acid should be changed to kainic acid
We thank the reviewer for catching this typo. The term “kainite acid” has been corrected to “kainic acid” in the revised manuscript.
Reviewer #2 (Recommendations For The Authors):
- Discuss limitations about the use of non-neuronal cells or cultured primary neurons rather than a more intact system.
We thank the reviewer for raising this point. We have discussed the limitations about the use of non-neuronal cells or cultured primary neurons rather than a more intact system (line 344 to line 348).
- Riluzole is not a selective drug, so the limitations of this drug should be discussed.
We thank the reviewer for raising this point. We have discussed the limitations of riluzole in the revised manuscript (line 360 to line 364).
- Remove the term in vivo.
We thank the reviewer for raising this point. In our experiments, although we did not conduct experiments directly in living organisms, our results demonstrated the coimmunoprecipitation of NaV1.6 with Slack in homogenates from mouse cortical and hippocampal tissues (Fig. 3C). This result may support that the interaction between Slack and NaV1.6 occurs in vivo.
- Figure 1
①C Why does Nav1.2 have a small inward current before the large inward current in the inset?
The slope of the rising phase of the larger sodium current seems greater than Nav1.6 or Nav1.5. Was this examined?
We apologize for the confusion. We would like to clarify that the small inward current can be attributed to the current of membrane capacitance (slow capacitance or C-slow). The larger inward current is mediated by NaV1.2. Additionally, we did not compare the slope of the rising phase of NaV subtypes sodium currents but primarily focused on the current amplitudes.
②D-E
For Nav1.5 the sodium current is very large compared to Nav1.6. Is it possible the greater effect of quinidine for Nav1.6 is due to the lesser sodium current of Nav1.6?
We thank the reviewer for raising this point. We would like to clarify that our results indicate that transient sodium currents contribute to the sensitization of Slack to quinidine blockade (Fig. 2C,E). Therefore, it is unlikely that the greater effect observed for NaV1.6 in sensitizing Slack is due to its lower sodium currents.
③The differences between WT and KO in G -H are hard to appreciate. Could quantification be shown? The text uses words like "block" but this is not clear from the figure. It seems that the replacement of Na+ with Li+ did not block the outward current or effect of quinidine.
We apologize for the confusion. We would like to clarify the methods used in this experiment. The lithium ion (Li+) is a much weaker activator of sodium-activated potassium channel Slack than sodium ion (Na+)1,2.
Zhang Z, Rosenhouse-Dantsker A, Tang QY, Noskov S, Logothetis DE. The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels. J Neurosci. Jun 2 2010;30(22):7554-62. doi:10.1523/JNEUROSCI.0525-10.2010
Kaczmarek LK. Slack, Slick and Sodium-Activated Potassium Channels. ISRN Neurosci. Apr
18 2013;2013(2013)doi:10.1155/2013/354262
Therefore, we replaced Na+ with Li+ in the bath solution to measure the current amplitudes of sodium-activated potassium currents (IKNa)3.
- Budelli G, Hage TA, Wei A, et al. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci. Jun 2009;12(6):745-50.
doi:10.1038/nn.2313
The following equation was used for quantification:
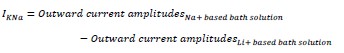
Furthermore, the remaining IKNa after application of 3 μM quinidine in the bath solution was measured as the following:

The quantification results were presented in Fig. 1K. The term "block" used in the text referred to the inhibitory effect of quinidine on IKNa.
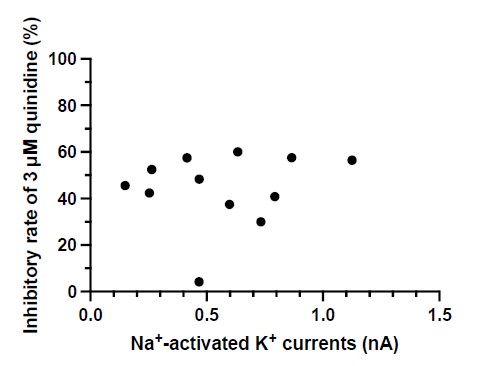
④In K, for the WT, why is the effect of quinidine only striking for the largest currents?
We thank the reviewer for raising this point. After conducting an analysis, we found no correlation between the inhibitory effect of quinidine and the amplitudes of baseline IKNa in WT neurons (p = 0.6294) (Author response image 2). Therefore, the effect of quinidine is not solely limited to targeting the larger currents.
Author response image 2.
The correlation between the inhibitory effect of quinidine and the amplitudes of baseline IKNa in WT neurons (data from manuscript Fig. 1K). r = 0.1555, p=0.6294,
Pearson correlation analysis.
- Figure 2
①A. The argument could be better made if the same concentration of quinidine were used for Slack and Slack + Nav1.6. It is recognized a greater sensitivity to quinidine is to be shown but as presented the figure is a bit confusing.
We apologize for the confusion. We would like to clarify that the presented concentrations of quinidine were chosen to be near the IC50 values for Slack and Slack+NaV1.6.
②C. Can the authors add the effect of quinidine to the condition where the prepulse potential was - 90?
We apologize for the confusion. We would like to clarify that the condition of prepulse potential at -90 mV is the same as the condition in Fig. 1. We only changed one experiment condition where the prepulse potential was changed to -40 mV from -90 mV.
- Figure 3.
①line 80 should be coronal not coronary
We thank the reviewer for catching this error. We have corrected the term “coronary” to
“coronal” in the caption of Figure 3.
②A. Clarify these 6 panels.
We thank the reviewer for raising this point. We have clarified the captions of Fig. 3A in the revised manuscript.
③Please enlarge fonts in D.
We thank the reviewer’s suggestion. We’ve enlarged the fonts in Fig. 3D in the revised manuscript.
④F. The variances should be checked with a test to determine if they are significantly different because they look different - if so, data can be transformed and if transformed data have variances that are equivalent a t-test can be used on the transformed data. Otherwise, Mann-Whitney should be used.
We thank the reviewer for pointing this out. We have reanalyzed the data in Fig. 3F using
Mann Whitney test after identifying the different variances in the two groups.
- Figure 7. The images need more clarity. They are very hard to see. Text is also hard to see.
We apologize for the lack of clarity in the images and text. we would like to provide a concise summary of the key findings shown in this figure.
Figure 7 illustrates an innovative intervention for treating SlackG269S-induced seizures in mice by disrupting the Slack-NaV1.6 interaction. Our results showed that blocking NaV1.6-mediated sodium influx significantly reduced Slack current amplitudes (Fig. 2D,G), suggesting that the Slack-NaV1.6 interaction contributes to the current amplitudes of epilepsy-related Slack mutant variants, aggravating the gain-of-function phenotype. Additionally, Slack’s C-terminus is involved in the Slack-NaV1.6 interaction (Fig. 5D). We assumed that overexpressing Slack’s C-terminus can disrupt the Slack-NaV1.6 interaction (compete with Slack) and thereby encounter the current amplitudes of epilepsy-related Slack mutant variants.
In HEK293 cells, overexpression of Slack’s C-terminus indeed significantly reduced the current amplitudes of epilepsy-related SlackG288S and SlackR398Q upon co-expression with NaV1.5/6NC (Fig. 7A,B). Subsequently, we evaluated this intervention in an in vivo epilepsy model by introducing the Slack G269S variant into C57BL/6N mice using AAV injection, mimicking the human Slack mutation G288S that we previously identified (Fig. 7C-G).
②It is not clear how data were obtained because injection of kainic acid does not lead to a convulsive seizure every 10 min for several hours, which is what appears to be shown. Individual seizures are just at the beginning and then they merge at the start of status epilepticus. After the onset of status epilepticus the animals twitch, have varied movements, sometime rear and fall, but there is not a return to normal behavior. Therefore one can not call them individual seizures. In some strains of mice, however, individual convulsive seizures do occur (even if the EEG shows status epilepticus is occurring) but there are rarely more than 5 over several hours and the graph has many more. Please explain.
We apologize for the confusion. Regarding the data acquisition in relation to kainic acid injection, we initiated the timing following intraperitoneal injection of kainic acid and recorded the seizure scores of per mouse at ten-minute intervals, following the methodology described in previous studies4.
- Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. Sep 2 2009;29(35):10979-88.
doi:10.1523/JNEUROSCI.1531-09.2009
The seizure scores were determined using a modified Racine, Pinal, and Rovner scale5,6: (1) Facial movements; (2) head nodding; (3) forelimb clonus; (4) dorsal extension (rearing); (5) Loss of balance and falling; (6) Repeated rearing and failing; (7) Violent jumping and running; (8) Stage 7 with periods of tonus; (9) Dead.
Pinel JP, Rovner LI. Electrode placement and kindling-induced experimental epilepsy. Exp
Neurol. Jan 15 1978;58(2):335-46. doi:10.1016/0014-4886(78)90145-0
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. Mar 1972;32(3):281-94. doi:10.1016/0013-
4694(72)90177-0
- The graphical abstract is quite complicated and somewhat hard to follow. Please simplify and clarify. One aspect of the abstract to clarify is the direction of what is first and second and third (etc.) because arrows point to many directions.
We thank the review for raising this point. In the revised manuscript, we have included numbering of three components within the graphical abstract:
Pathological phenotype: Increased Slack currents.
Two types of interventions:
2a. Disruption of the Slack-NaV1.6 interaction.
2b. NaV1.6-mediated sensitization of Slack to quinidine blockade.
- Therapeutic effects: Reduced Slack currents.
Reviewer #3 (Recommendations For The Authors):
- A reference to homozygous knockout is made in the abstract; however, only heterozygous mice are mentioned in the methods section. The genotype of the mice needs to be made clear in the manuscript. Furthermore, at what age were these mice used in the study. Since homozygous knockout of NaV1.6 is lethal at a very young age (<4 wks), it would be important to clarify that point as well.
We thank the reviewer for pointing this out. In the revised manuscript, we have included information about the source of the primary cortical neurons used in our study. These neurons were obtained from postnatal homozygous NaV1.6 knockout C3HeB/FeJ mice and their wild-type littermate controls.
- Coimmunoprecipitation studies in Fig. 3C are not convincing. There appears to be a signal in the control lane. Furthermore, it appears that brightness levels were adjusted of that image, thereby removing completely the background.
We thank the reviewer for pointing this out. We have replaced Fig. 3C with an unadjusted version in the revised manuscript.
- In Fig. 1B, the authors indicate that 30 microM of quinidine was used, while the corresponding figure legend suggest that 1 microM. Please clarify.
We apologize for this error. We have corrected the concentration value in the caption of
Figure 1 from "1 μΜ" to "30 μΜ" in the revised manuscript.
- How long were the cells exposed to quinidine before the functional measurement were performed?
We thank the reviewer for pointing this out. The cells were exposed to the bath solution with quinidine for about one minute before applying step pulses.
- In Fig. 6B-D, it is not clear to what extent co-expression of Slack mutants and NaV1.6 increases sodium-activated potassium current.
We thank the reviewer for pointing this out. We notice that the current amplitudes of Slack mutants exhibit a considerable degree of variation, ranging from less than 1 nA to over 20 nA (n = 5-8). To accurately measure the effects of NaV1.6 on increasing current amplitudes of Slack mutants, we plan to apply tetrodotoxin in the bath solution to block NaV1.6 sodium currents upon coexpression of Slack mutants with NaV1.6.
- In Fig.7A and B, it appears that some recordings had no sodium-activated potassium currents. Why were these included in analysis? How was transfection efficacy assessed?
We apologize for the confusion. We would like to clarify that all recordings included in analysis indeed exhibited outward sodium-activated potassium currents. The current density data in
Fig. 7A-B are listed in Author response table 1 (in pA/pF):
Author response table 1.
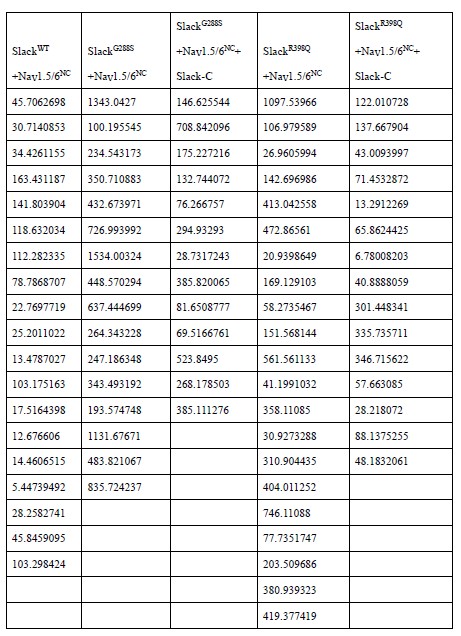
Regarding the assessment of transfection efficacy, we estimated it approximately by using fluorescence proteins as reporters, which were co-expressed with the relevant proteins via the selfcleaving 2A peptide.
- Greater detail needs to be provided for the generation of NaV1.5 and NaV1.6 chimeras.
Specifically, what AA residues were changed between sodium channel isoforms?
We thank reviewer for pointing this out. In the revised manuscript, we have included the specific amino acid residues that were changed between NaV1.5 and NaV1.6 to generate the chimeric constructs.
- In line 481, the authors refer to Fig. S2d instead of Fig. S6D. This should be corrected. Furthermore, the unusual shift in sodium current kinetics that the authors observe might be due in part to junction potential. Did the authors take that into consideration?
We apologize for this error. The reference to "Fig. S2d" has been corrected to "Fig. S6D" in the revised manuscript.
Regarding the unusual shift observed in the sodium current kinetics, we agree with the reviewer's suggestion that the junction potential may contribute to this phenomenon. During patch-clamp recordings, we ensure that the junction potential was properly compensated by the amplifier. Additionally, the replacement of CsF in pipette solution may have contributed to the observed unusual shift, as CsF in pipette solution has been reported to shift the voltage dependence of activation and fast/slow inactivation of NaV channels towards more negative potentials7.
- Korngreen A. Advanced patch-clamp analysis for neuroscientists. Neuromethods. Humana
Press; 2016:xii, 350 pages.
- Legends for Fig.S6E and S6F are flipped. Please correct.
We apologize for this error. We have rectified the flipped captions for figure S6E and S6F in the revised manuscript.
- Variance should be provided for the IC50 values and kinetic parameters of the sodium channels in the supplemental tables.
We thank the reviewer for raising this point. We have included the 95% confidence interval
(95%CI) for the IC50 values and kinetic parameters in the revised supplementary tables.
Additionally, we have corrected some equations in the methods section:
Line 500 and line 503: We have corrected equation (1) by adding the parameter hill coefficient.
Line 514: We have revised equation (4) from 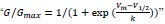 to
to