Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorMarisa BartolomeiUniversity of Pennsylvania, Philadelphia, United States of America
- Senior EditorWei YanWashington State University, Pullman, United States of America
Reviewer #1 (Public Review):
The work by Debashish U. Menon, Noel Murcia, and Terry Magnuson brings important knowledge about histone H3.3 dynamics involved in meiotic sex chromosome inactivation (MSCI). MSCI is unique to gametes and failure during this process can lead to infertility. Classically, MSCI has been studied in the context of DNA Damage repair pathways and little is known about the epigenetic mechanisms behind maintenance of the sex body as a silencing platform during meiosis. One of the major strengths of this work is the evidence provided on the role of ARID1A, a BAF subunit, in MSCI through the regulation of H3.3 occupancy in specific genic regions.
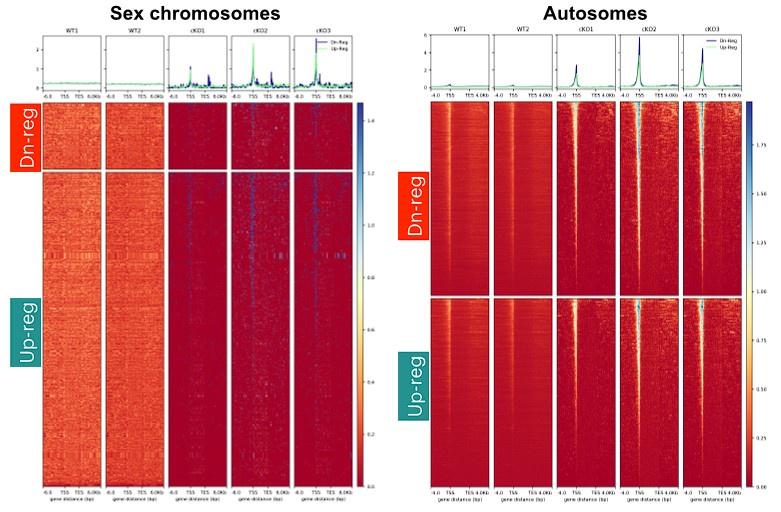
Using RNA seq and CUT&RUN and ATAC-seq, the authors show that ARID1A regulates chromatin accessibility of the sex chromosomes and XY gene expression. Loss of ARID1A increases promoter accessibility of XY linked genes with concomitant influx of RNA pol II to the sex body and up regulation of XY-linked genes. This work suggests that ARID1A regulates chromatin composition of the sex body since in the absence of ARID1A, spermatocytes show less enrichment of H3.3 in the sex chromosomes and stable levels of the canonical histones H3.1/3.2. By overlapping CUT&RUN and ATAC-seq data, authors show that changes in chromatin accessibility in the absence of ARID1A are given by redistribution of occupancy of H3.3. Gained open chromatin in mutants corresponds to up regulation of H3.3 occupancy at transcription start sites of genes mediated by ARID1A.
Interestingly, ARID1A loss caused increased promoter occupancy by H3.3 in regions usually occupied by PRDM9. PRDM9 catalyzes histone H3 lysine 4 trimethylation during meiotic prophase I, and positions double strand break (DSB) hotspots. Lack of ARID1A causes reduction in occupancy of DMC1, a recombinase involved in DSB repair, in non-homologous sex regions. These data suggest that ARID1A might indirectly influence DNA DSB repair on the sex chromosomes by regulating the localization of H3.3. This is very interesting given the recently suggested role for ARID1A in genome instability in cancer cells. It raises the question of whether this role is also involved in meiotic DSB repair in autosomes and/or how this mechanism differs in sex chromosomes compared to autosomes.
The fact that there are Arid1a transcripts that escape the Cre system in the Arid1a KO mouse model might difficult the interpretation of the data. The phenotype of the Arid1a knockout is probably masked by the fact that many of the sequencing techniques used here are done on a heterogeneous population of knockout and wild type spermatocytes. In relation to this, I think that the use of the term "pachytene arrest" might be overstated, since this is not the phenotype truly observed. Nonetheless, the authors provide evidence showing that the spermatids observed in cKO testes that progress in spermatogenesis are the ones expressing Arid1a. This work presents enough evidence to include the BAF complex as part of the MSCI process, which increases our knowledge on specific regulation of the sex chromatin during meiosis.
Reviewer #2 (Public Review):
The authors tried to characterize the function of the SWI/SNF remodeler family, BAF, in spermatogenesis. The authors focused on ARID1A, a BAF-specific putative DNA binding subunit, based on gene expression profiles.
The authors disagreed with my previous assessments. I disagree with their response.
Reviewer #3 (Public Review):
In this manuscript, Magnuson and colleagues investigate the meiotic functions of ARID1A, a putative DNA binding subunit of the SWI/SNF chromatin remodeler BAF. The authors develop a germ cell specific conditional knockout (cKO) mouse model using Stra8-cre and observe that ARID1A-deficient cells fail to progress beyond pachytene, although due to inefficiency of the Stra8-cre system the mice retain ARID1A-expressing cells that yield sperm and allow fertility. Because ARID1A was found to accumulate at the XY body late in Prophase I, the authors suspected a potential role in meiotic silencing and by RNAseq observe significant misexpression of sex-linked genes that typically are silenced at pachytene. They go on to show that ARID1A is required for exclusion of RNA PolII from the sex body and for limiting promoter accessibility at sex-linked genes, consistent with a meiotic sex chromosome inactivation (MSCI) defect in cKO mice. The authors proceed to investigate the impacts of ARID1A on H3.3 deposition genome-wide. H3.3 is known be regulated by ARID1A and is linked to silencing, and here the authors find that upon loss of ARID1A, overall H3.3 enrichment at the sex body as measured by IF failed to occur, but H3.3 was enriched specifically at transcriptional start sites of sex-linked genes that are normally regulated by ARID1A. The results suggest that ARID1A normally prevents H3.3 accumulation at target promoters on sex chromosomes and based on additional data, restricts H3.3 to intergenic sites. Finally, the authors present data implicating ARID1A and H3.3 occupancy in DSB repair, finding that ARID1A cKO leads to a reduction in focus formation by DMC1, a key repair protein. Overall the paper provides new insights into the process of MSCI from the perspective of chromatin composition and structure, and raises interesting new questions about the interplay between chromatin structure, meiotic silencing and DNA repair.
In general the data are convincing. The conditional KO mouse model has some inherent limitations due to incomplete recombination and the existence of 'escaper' cells that express ARID1A and progress through meiosis normally. This reviewer feels that the authors have addressed this point thoroughly and have demonstrated clear and specific phenotypes using the best available animal model. The data demonstrate that the mutant cells fail to progress past pachytene, although it is unclear whether this specifically reflects pachytene arrest, as accumulation in other stages of Prophase also is suggested by the data in Table 1.
The revised manuscript more appropriately describes the relationship between ARID1A and DNA damage response (DDR) signaling. The authors don't see defects in a few DDR markers in ARID1A CKO cells (including a low resolution assessment of ATR), suggesting that ARID1A may not be required for meiotic DDR signaling. However, as previously noted the data do not rule out the possibility that ARID1A is downstream of DDR signaling, and the authors note the possibility of a role for DDR signaling upstream of ARID1A.
A final comment relates to the impacts of ARID1A loss on DMC1 focus formation and the interesting observation of reduced sex chromosome association by DMC1. The authors additionally assess the related recombinase RAD51 and suggest that it is unaffected by ARID1A loss. However, only a single image of RAD51 staining in the cKO is provided (Fig. S11) and there are no associated quantitative data provided. The data are suggestive and conclusions about the impacts of ARID1A loss on RAD51 must be considered as preliminary until more rigorously assessed.