Author response:
The following is the authors’ response to the original reviews.
Reviewer #1:
Detection of early-stage colorectal cancer is of great importance. Recently, both laboratory scientists and clinicians have reported different exosomal biomarkers to identify colorectal cancer patients.
Here, the authors exhibited a full RNA landscape for plasma exosomes of 60 individuals, including 31 colorectal cancer (CRC) patients, 19 advanced adenoma (AA) patients, and 10 noncancerous controls. RNAs with high fold change, high absolute abundance, and various module attribution were used to construct RT-qPCR-based RNA models for CRC and AA detection.
Overall, this is a well-performed proof-of-concept study to highlight exosomal RNAs as potential biomarkers of early-stage colorectal cancer and its precancerous lesions.
Thank you for your careful evaluation and valuable suggestions, which have provided valuable guidance for the improvement of our paper. In response to your feedback, we have implemented the following improvements.
(1) Depicting the full RNA landscape of circulating exosomes is still quite challenging. The authors annotated 58,333 RNA species in exosomes, most of which were lncRNAs, but the authors do not explain how they characterized those RNAs.
Author response and action taken: Thanks for your comments. In the Supplementary Methods section titled "Identification of mRNAs and lncRNAs", we have provided a comprehensive explanation on the characterization of mRNAs and lncRNAs to address the concerns you raised. Characterization of long-chain RNAs is a great challenge. For lncRNA analysis, the transcriptome was assembled using the Cufflinks and Scripture based on the reads mapped to the reference genome. The assembled transcripts were annotated using the Cuffcompare program from the Cufflinks package. The unknown transcripts were used to screen for putative lncRNAs.
(2) The authors tested their models in a medium size population of 124 individuals, which is not enough to obtain an accurate evaluation of the specificity and sensitivity of the biomarkers proposed here. External validation would be required.
Author response and action taken: Thanks for your comments. We fully acknowledge the significance of external validations in the evaluation of diagnostic model performance. Unfortunately, as a pilot study, we currently do not have the conditions for a multicenter investigation. To mitigate result bias and overfitting effects, we implemented a rigorous variable selection strategy and enhanced model stability through 10-fold cross-validation. In the meantime, we will persist in our efforts to elevate the quality of our research and seek additional resources for external validation in future studies.
Reviewer #2:
The authors present an important study on the potential of small extracellular vesicle (sEV)-derived RNAs as biomarkers for the early detection of colorectal cancer (CRC) and precancerous adenoma (AA). The authors provide a detailed analysis of the RNA landscape of sEVs isolated from participants, identifying differentially expressed sEV-RNAs associated with T1a stage CRC and AA compared to normal controls. The paper further categorises these sEV-RNAs into modules and constructs a 60-gene model that successfully distinguishes CRC/AA from NC samples. The authors also validate their findings using RT-qPCR and propose an optimised classifier with high specificity and sensitivity. Additionally, the authors discuss the potential of sEV-RNAs in understanding CRC carcinogenesis and suggest that a comprehensive biomarker panel combining sEV-RNAs and proteins could be promising for identifying both early and advanced CRC patients. Overall, the study provides valuable insights into the potential clinical application of sEV-RNAs in liquid biopsy for the early detection of CRC and AA.
Major strengths:
(1) Comprehensive sEV RNA profiling: The study provides a valuable dataset of the whole-transcriptomic profile of circulating sEVs, including miRNA, mRNA, and lncRNA. This approach adds to the understanding of sEV-RNAs' role in CRC carcinogenesis and facilitates the discovery of potential biomarkers.
(2) Detection of early-stage CRC and AA: The developed 60-gene t-SNE model successfully differentiated T1a stage CRC/AA from normal controls with high specificity and sensitivity, indicating the potential of sEV-RNAs as diagnostic markers for early-stage colorectal lesions.
(3) Independent validation cohort: The study combines RNA-seq, RT-qPCR, and modelling algorithms to select and validate candidate sEV-RNAs, maximising the performance of the developed RNA signature. The comparison of different algorithms and consideration of other factors enhance the robustness of the findings.
Thank you for your careful evaluation and valuable suggestions. These comments have been highly valuable for the performance evaluation and clinical applications of our work. In response to your feedback, we have implemented the following improvements.
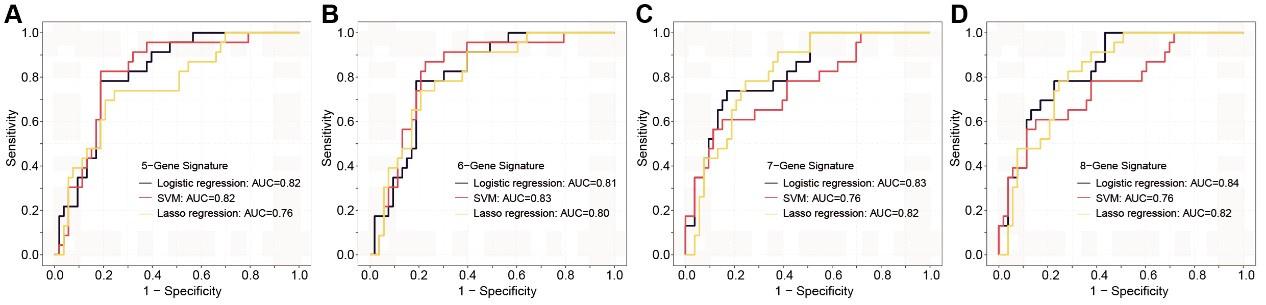
(1). Lack of analysis on T1-only patients in the validation cohort: While the study identifies key sEV-RNAs associated with T1a stage CRC and AA, the validation cohort is only half of the patients in T1(25 out of 49). It would be better to do an analysis using only the T1 patients in the validation cohort, so the conclusion is not affected by the T2-T3 patients.
Author response and action taken: Thanks for your comments. This feedback is essential for ensuring consistency in the results with our previous findings. In this context, we revalidated various diagnostic panels using exclusively Stage I patients (Figure 7—figure supplement 2). To minimize the potential overfitting effect due to the reduction in sample size after partitioning, we implemented a 10-fold cross-validation for each panel and these panels exhibit promising performance in Stage I colorectal cancer (CRC) patients.
Author response image 1.
The ROC analysis of different sEV-RNA signatures in the prediction of Stage I CRC patients by different algorithms (a: 6-gene panel; b: 7-gene panel; c: 8-gene panel; d: 9-gene panel).
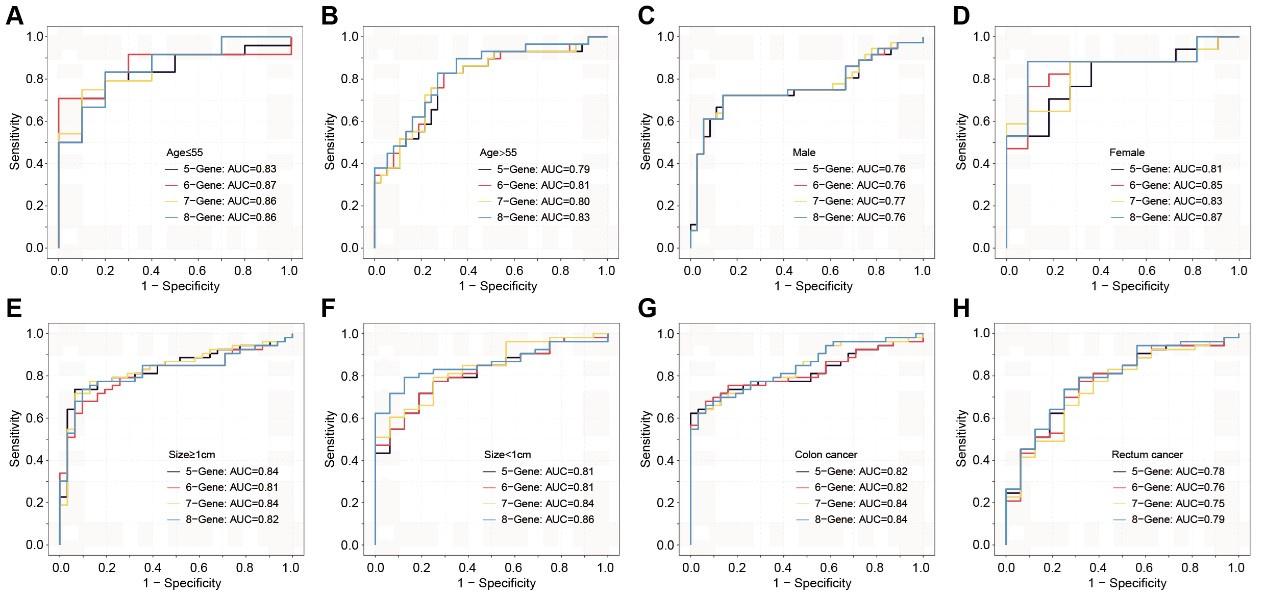
(2). Lack of performance analysis across different demographic and tumor pathology factors listed in Supplementary Table 12. It's important to know if the sEV-RNAs identified in the study work better/worse in different age/sex/tumor size/Yamada subtypes etc.
Author response and action taken: Thanks for your comments. This feedback will be immensely beneficial for clinical diagnosis. Similarly, cross-validation was performed in this section. We assessed the discriminative effects of CRC on NC, taking into account different age groups, genders, tumor sizes, and anatomical locations (Figure 7—figure supplement 3). Overall, these sEV RNA panels perform better in individuals under the age of 55 and in female patients. There is no significant difference in discriminative effects across different tumor sizes. Compared to rectal cancer, the discriminative effects are better in colon cancer.
Author response image 2.
The ROC analysis of different sEV-RNA signatures for predicting CRC patients using the Lasso regression algorithm in different clinical parameters (ab: age; cd: gender; ef: tumor size; gh: anatomical position).