Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorPeter TurnbaughUniversity of California, San Francisco, San Francisco, United States of America
- Senior EditorWendy GarrettHarvard T.H. Chan School of Public Health, Boston, United States of America
Reviewer #1 (Public Review):
The major strength of this work is its scope, including detailed mouse phenotyping, inter-disciplinary methods, and numerous complementary experiments. The antibiotic depletion and FMT experiments provide support for a role of the gut microbiota in this mouse model.
A major limitation is the lack of studies narrowing down which microbes are responsible. Sequencing data is shown, but no follow-up studies are done with bacterial isolates or defined communities.
The link to GABA is also somewhat tenuous. While it does match the phenotypic data, there are no targeted experiments in which GABA producing microbial communities/strains are compared to a control community/strain. As such, it seems difficult to know how much of the effects in this model are due to GABA vs. other metabolites.
My major recommendation would be to revise the title, abstract, and discussion to provide more qualification and to consider alternative interpretations.
Some key controls are also missing, which could be addressed by repeat experiments in the mouse model. The antibiotic depletion experiment would be improved by testing the effect of antibiotics in the absence of metformin, to see if the effect is just driven by the model itself as opposed to an interaction between metformin and antibiotics. The FMT experiment lacks a control group and suffers from pseudoreplication: multiple donors from metformin treated and untreated mice could be used to colonize separate groups of recipient mice.
Reviewer #2 (Public Review):
The authors examine the use of metformin in the treatment of hepatic ischemia/reperfusion injury (HIRI) and suggest the mechanism of action is mediated in part by the gut microbiota and changes in hepatic ferroptosis. While the concept is intriguing, the experimental approaches are inadequate to support these conclusions.
The histological and imaging studies were considered a strength and reveal a significant impact of metformin post-HIRI.
Weaknesses largely stem from the experimental design. The impact of metformin on the microbiota is profound resulting in changes in bile acid, lipid, and glucose homeostasis. Throughout the manuscript no comparisons are made with metformin alone which would better capture the metformin-specific effects. With the pathology and metabolic disturbances resulting from HIRI, it is important to understand if metformin is providing beneficial effects from reported mechanisms such as changes in bile acid, glucose, and/or lipid metabolism, or are these changes the result of a new unappreciated mechanism. A comparison of the reported and the new pathways is not included.
Overall, while the concept is interesting and has potential to better understand the pleiotropic functions of metformin, the limitations with the experimental design and lack of key controls make it challenging to support the conclusions.
Author Response
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Many drugs have off-target effects on the gut microbiota but the downstream consequences for drug efficacy and side effect profiles remain unclear. Herein, Wang et al. use a mouse model of liver injury coupled to antibiotic and microbiota transplantation experiments. Their results suggest that metformin-induced shifts in gut microbial community structure and metabolite levels may contribute to drug efficacy. This study provides valuable mechanistic insights that could be dissected further in future studies, including efforts to identify which specific bacterial species, genes, and metabolites play a causal role in drug response. Importantly, although some pilot data from human subjects is shown, the clinical relevance of these findings for liver disease remain to be determined.
Thank you for reviewing our manuscript. We appreciate your valuable feedback. We agree that the downstream consequences of off-target effects on the gut microbiota by various drugs remain unclear. Our study aimed to shed light on this aspect by utilizing a mouse model of liver injury and conducting antibiotic and microbiota transplantation experiments. Our findings suggest that shifts in the structure and metabolite levels of the gut microbial community induced by metformin play a role in the drug’s efficacy. We believe that these mechanistic insights provide a strong foundation for further investigations. Specifically, future studies could focus on identifying the specific bacterial species, genes, and metabolites that have a causal role in drug response. While we have included some pilot data from human subjects, we acknowledge that the clinical relevance of our findings in the context of liver disease still requires further determination. In fact, we focused on the alteration of microbiota and metabolism caused by metformin in human bodies, which could capture the characteristics of changes in a more composite clinical direction, elucidating the potential role of metformin. We appreciate your attention to this aspect and thank you again for your thoughtful review and valuable suggestions.
The major strength of this work is its scope, including detailed mouse phenotyping, inter-disciplinary methods, and numerous complementary experiments. The antibiotic depletion and FMT experiments provide support for a role of the gut microbiota in this mouse model.
A major limitation is the lack of studies narrowing down which microbes are responsible. Sequencing data is shown, but no follow-up studies are done with bacterial isolates or defined communities.
We acknowledge the limitation of our study in not narrowing down the specific microbes responsible for the observed effects. We hold the opinion that metformin exerts its effects through modulation of specific metabolic pathways unique to the microbial community. Previous study has shown that metformin can inhibit microbial folate metabolism, leading to longevity-promoting effects that are not attributed to a single colony or strain[1]. Similarly, the impact of metformin on amino acid metabolism in the microbial community appears to be widespread. While further investigations with bacterial isolates or defined communities are needed, our findings suggest that metformin's effects on microbial metabolism are complex and involve multiple members of the microbial community.
The link to GABA is also somewhat tenuous. While it does match the phenotypic data, there are no targeted experiments in which GABA producing microbial communities/strains are compared to a control community/strain. As such, it seems difficult to know how much of the effects in this model are due to GABA vs. other metabolites.
We agree with your point regarding the tenuous link to GABA in our study. While we did observe an increase in GABA as the only amino acid following metformin treatment, and this finding has not been reported previously, we acknowledge the need for targeted experiments comparing GABA-producing microbial communities/strains to control communities/strains. Previous literatures suggest that metformin's modulation of the microbiota can vary significantly depending on the disease context, with different microbial populations exhibiting differential responses[2-4]. Given this complexity, we opted to study the overall microbial community response to metformin rather than focusing on specific strains. Additionally, our detection of key enzymes involved in GABA synthesis at the community level further supports our findings.
My major recommendation would be to revise the title, abstract, and discussion to provide more qualification and to consider alternative interpretations.
We appreciate your feedback and understand your concern regarding the need for more qualification and consideration of alternative interpretations. We hope to have more specific and detailed suggestions you may have to enhance the clarity and qualification of our title and abstract. Furthermore, we have tried to revise discussion in order to enhance the scientific rigor and logical coherence of our study. If you have any specific recommendations or insights, we would be more than willing to make further revisions to address those concerns.
Some key controls are also missing, which could be addressed by repeat experiments in the mouse model.
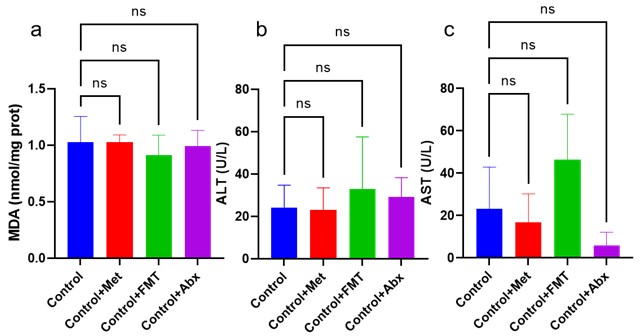
We appreciate your suggestion to include additional key controls in the mouse model experiments. We have conducted repeat experiments to test the effect of antibiotics in the absence of metformin to differentiate between the effects of the model itself and the interaction of metformin with antibiotics. As results of liver injury indicators shown, there were no significance among Control, Control+Met, Control+FMT and Control+Abx groups, revealing that metformin and its treated feces, and antibiotics had no effect on liver function in normal mice (Figure 1).
Author response image 1.
Figure1 a: Liver MDA detection; b: Serum ALT level; c: Serum AST level.
The antibiotic depletion experiment would be improved by testing the effect of antibiotics in the absence of metformin, to see if the effect is just driven by the model itself as opposed to an interaction between metformin and antibiotics.
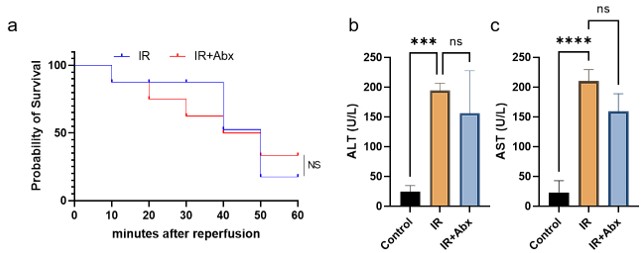
For the antibiotic depletion experiment, we had used antibiotics (Abx) for the mice of modeling, and the survival rate and liver function detection suggested that Abx had no extra effect on liver, which demonstrated that the effect is just driven by the model itself as opposed to an interaction between metformin and antibiotics (Figure 2).
Author response image 2.
Figure2 a: Survival rate between IR and IR + Abx group; b: Serum ALT level; c: Serum AST level.
References
[1] CABREIRO F, AU C, LEUNG K Y, et al. Metformin Retards Aging in C. elegans by Altering Microbial Folate and Methionine Metabolism [J]. Cell, 2013, 153(1): 228-39.
[2] LIANG H, SONG H, ZHANG X, et al. Metformin attenuated sepsis-related liver injury by modulating gut microbiota [J]. Emerg Microbes Infect, 2022, 11(1): 815-28.
[3] SUN L, XIE C, WANG G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin [J]. Nat Med, 2018, 24(12): 1919-29.
[4] ZHAO H Y, LYU Y J, ZHAI R Q, et al. Metformin Mitigates Sepsis-Related Neuroinflammation via Modulating Gut Microbiota and Metabolites [J]. Frontiers in Immunology, 2022, 13:797312.
Reviewer #2 (Public Review):
The authors examine the use of metformin in the treatment of hepatic ischemia/reperfusion injury (HIRI) and suggest the mechanism of action is mediated in part by the gut microbiota and changes in hepatic ferroptosis. While the concept is intriguing, the experimental approaches are inadequate to support these conclusions.
The histological and imaging studies were considered a strength and reveal a significant impact of metformin post-HIRI.
Thank you for reviewing our paper titled “Gut microbiota-derived gamma-aminobutyric acid from metformin treatment reduces hepatic ischemia/reperfusion injury through inhibiting ferroptosis”. We appreciate your insightful comments and suggestions, which have provided valuable insights into improving the quality and credibility of my research. We agree with your assessment that the experimental approaches used in this study may have limitations in supporting the conclusions drawn, and we appreciate your recognition of the strength of our histological and imaging studies, which clearly demonstrate the impact of metformin post-HIRI.
Weaknesses largely stem from the experimental design. First, use of the iron chelator DFO would be strengthened using the ferroptosis inhibitor, liproxstatin.
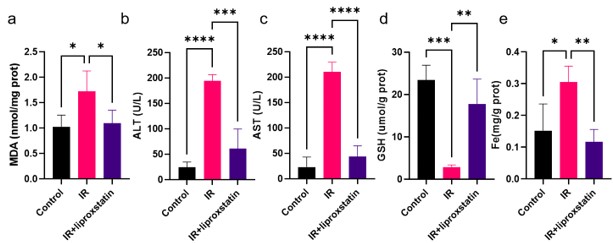
Your suggestion to employ the ferroptosis inhibitor, liproxstatin, in addition to the iron chelator DFO is well-taken. Incorporating liproxstatin into our experimental setup would provide a more comprehensive understanding of the involvement of hepatic ferroptosis in the mechanism of action of metformin. Therefore, we employed liproxstatin to inhibit HIRI and detected some core indicators of liver injury. As figure 3 shown, liproxstatin can reduce liver injury, restore liver GSH level and inhibit Fe accumulation, suggesting that ferroptosis plays an important role in HIRI. We hope this modification will enhance the credibility of our conclusions.
Author response image 3.
Figure3 a: Liver MDA detection; b: Serum ALT level; c: Serum AST level; d: Liver GSH level; e: Liver Fe level.
Second, the impact of metformin on the microbiota is profound resulting in changes in bile acid, lipid, and glucose homeostasis. Throughout the manuscript no comparisons are made with metformin alone which would better capture the metformin-specific effects.
Thank you for raising an important point regarding the impact of metformin on the microbiota and its potential effects on bile acid, lipid, and glucose homeostasis. It has well known that that the effects of metformin on normal blood glucose and lipid metabolism are minimal. Metformin primarily exerts its effects in cases of impaired glucose tolerance, which is why it is widely used for non-diabetic conditions. Regarding the changes in bile acid metabolism and chronic cholesterol and lipid elevation, these associations are typically observed in chronic liver disease models. Since our study focuses on an acute model of HIRI, we did not specifically investigate these changes.
Lastly, the absence of proper controls including germ free mice, metformin treated mice, FMT treated mice, etc make it difficult to understand the outcomes and to properly reproduce the findings in other labs.
Lastly, we acknowledge your concern regarding the absence of proper controls, including germ-free mice, metformin-treated mice, and FMT -treated mice. We understand that these controls are essential for robustly interpreting and reproducing our findings. Therefore, we have added a batch of experiments for verification. As results shown, there were no significance among Control, Control+Met, Control+FMT and Control+Abx groups, revealing that metformin and its treated feces, and antibiotics had no effect on liver function in normal mice (Figure 1). We hope the result of these controls could address your valid point and provide a more comprehensive framework for understanding the outcomes.
Author response image 4.
Figure1 a: Liver MDA detection; b: Serum ALT level; c: Serum AST level.
Overall, while the concept is interesting and has the potential to better understand the pleiotropic functions of metformin, the limitations with the experimental design and lack of key controls make it challenging to support the conclusions.
We genuinely appreciate your constructive criticism and the time you have taken to evaluate my work. Your feedback has shed light on the limitations of our experimental design and the need for key controls, which we have addressed in revised manuscript. If you have any further recommendations or concerns, we would be more than willing to incorporate them into my future work.
Reviewer #3 (Public Review):
The study presented in this paper explores the role of gut microbiota in the therapeutic effect of metformin on HIRI, as supported by fecal microbiota transplantation (FMT) experiments. Through high throughput sequencing and HPLC-MS/MS, the authors have successfully demonstrated that metformin administration leads to an increase in GABA-producing bacteria. Moreover, the study provides compelling evidence for the beneficial impact of GABA on HIRI.
Thank you for your valuable feedback on our paper exploring the role of gut microbiota in the therapeutic effect of metformin on hepatic ischemia-reperfusion injury (HIRI). We appreciate your positive remarks and suggestions for improvement. In response to your comments, we have revised the manuscript accordingly. We have included additional details on the high throughput sequencing and HPLC-MS/MS methods used to analyze the gut microbiota and GABA levels. This should provide readers with a clearer understanding of our experimental approach and the evidence supporting our findings.
Regarding your suggestion to further investigate the mechanisms underlying the beneficial impact of GABA on HIRI, we agree that this is an important direction for future research. We plan to conduct additional studies to explore the specific mechanisms by which GABA exerts its protective effects on HIRI in the future. We also supplemented discussion of potential therapeutic strategies targeting GABAergic pathways in the discussion section.
Thank you once again for your insightful comments. We believe that these revisions have strengthened the manuscript and improved its scientific rigor. We hope that you find the revised version to be satisfactory and look forward to your further feedback.
Reviewer #1 (Recommendations For The Authors):
The writing could be improved. Multiple typos are found throughout and there is an overuse of adverbs like "expectedly". You should let the reader decide what is or is not expected. Try to avoid terms like "confirmed" or "validated", which only applies if you knew the result a priori. Remove underscores in species names. The Results section is also very difficult to interpret given the lack of explanation of experimental design. For example, the human study is only briefly mentioned within a larger paragraph on mouse data, without any explanation as to the study design. Similar issues are true for the transcriptomics and amplicon sequencing - it would help the reader to explain what samples were processed, the timepoints, etc.
Thank you for your valuable feedback on our manuscript entitled “Gut microbiota-derived gamma-aminobutyric acid from metformin treatment reduces hepatic ischemia/reperfusion injury through inhibiting ferroptosis” We appreciate your constructive comments and insightful suggestions for improvement.
We have carefully reviewed your comments and have made several revisions to enhance the clarity and readability of the manuscript. We have addressed the issue of multiple typos and have removed the overuse of adverbs, such as “expectedly,” to allow readers to draw their own conclusions from the results. Additionally, we have eliminated terms like “confirmed” or “validated” that may imply a priori knowledge of the results.
We apologize for the lack of clarity regarding the experimental design in the Results section. We have now provided a more detailed explanation of the study design for the human study, transcriptomics, and amplicon sequencing experiments. This includes information on the samples processed, timepoints, and other relevant details, to aid readers in understanding the experimental procedures.
In response to your comment about removing underscores in species names, we have revised the text accordingly to ensure consistency and accuracy in the species nomenclature used throughout the manuscript.
Once again, we sincerely appreciate your valuable input, which has helped us improve the quality of our manuscript. We hope that the revised version now meets your expectations and look forward to any further feedback you may have.
Thank you for your time and attention.
Line 53 - prebiotics aren't "microbial agents"
We apologize for this error, which we have corrected. (line 55: “Microbial agents, such as synbioticsprebiotics and probiotics…”)
Line 88 - sequencing doesn't "verify the critical role of gut microbiota"
We apologize for this error, which we have corrected. (line 90: “In order to verifyclarify the critical role of gut microbiota in the pleiotropic actions of metformin,22-24 fecal samples were collected from the mice to perform 16S rRNA sequencing.
Line 92 - missing a citation for the "microbiota-gut-liver axis theory"
We have corrected it in manuscript. (line 93: “Next, as the microbiota-gut-liver axis theory indicates,25 HIRI-induced dysfunction of the gut barrier may aggravate liver damage by disrupting the gut microbiota.”)
Line 112 - it's very surprising to me that FMT led to lower alpha diversity, which seems impossible.
We understand your surprise regarding the observed decrease in alpha diversity after FMT. Our findings indeed deviate from the commonly observed pattern of increased alpha diversity post-FMT. We have carefully re-examined our data and conducted additional analyses to ensure the accuracy of our results. After thorough investigation, we have identified a potential reason for this unexpected outcome, which we believe could shed light on this phenomenon. We hypothesize that the lower alpha diversity observed in our study might be attributed to the specific characteristics of the donor microbiota used for FMT. While the donor microbiota exhibited certain beneficial properties associated with the therapeutic effect on HIRI, it could have presented a limited diversity compared to the recipient’s original gut microbiota. This discrepancy in diversity could have contributed to the observed decrease in alpha diversity following FMT.
To further support our hypothesis, we have included a discussion on this unexpected finding in the revised manuscript. We believe that this addition will provide a more comprehensive understanding of the results and help contextualize the observed decrease in alpha diversity following FMT.
Line 117 - Antibiotics don't "identify the function of gut microbes." Need to specify which antibiotics were used and for how long.
We have corrected it in manuscript. (line 119: “To further identify the function of gut microbes, experiments were designed, and combination treatment of antibiotics (1 mg/mL penicillin sulfate, 1 mg/mL neomycin sulfate, 1 mg/mL metronidazole and 0.16 mg/mL gentamicin) and metformin were employed for 1 week before IR treated.”)
Line 120 - this experiment shows that the gut microbiota (or antibiotics more precisely) matters, not the "reshaped gut microbiota"
We have corrected it in manuscript. (line 124: “The results confirmed that reshaped gut microbiota is critical for the effect of metformin against HIRI.”)
Line 122 - need to reword this subheading and the concluding sentence. The main takeaway is that the FMT improved markers of ferroptosis, but no additional causal links are provided here.
We have revised in manuscript. (line 125: “FMT alleviates HIRI-induced ferroptosis through reshaped fecal microbiota.”)
Line 141 - need to explain what transcriptomics data was generated and how it was analyzed.
We have revised in manuscript. (line 144: “To elucidate the molecular mechanisms through which pathway participates metformin-treated IR injury, we analysed gene expression profiles of each group mice. Transcriptome sequencing analysis revealed that 9697 genes were in common among four groups (Supplementary Figure 6). Therefore, we used these common genes for KEGG analysis, showing that The transcriptome analysis of liver tissues showed that similar mRNA changes between Met group and FMT group are mainly concentrated in the three top pathways: lipid metabolism, carbohydrate metabolism, and amino acid metabolism (Fig 4a).”)
Line 150 - change to "16S rRNA gene sequencing". Typo: "mice microbes".
We have revised in manuscript. (line 156: “Moreover, it was observed that the genus of Bacteroides had a significant increase based on the 16s rRNA gene sequencing of metformin-treated mice microbes.”)
Line 152 - upregulated refers to gene expression, change to enriched.
We have revised in manuscript. (line 171: “Detailedly, the species of Bacteroides containing Bacteroides thetaiotaomicron, Bacteroides unifomis, and Bacteroides salyersiae, were enriched in human gut after metformin administration (Fig. 4i).”)
Line 159 - typo: "prokaryotes"
We have revised in manuscript. (line 165: “In order to further identify the increased GABA originates from gut microbiota, two key enzymes of prokaryotes protokaryotic GABA synthesis, GAD and PAT, were detected on DNA level, finding that both of them are significantly increased in the feces from IR+Met and IR+FMT groups (Fig. 4h).”)
Line 161 - the human study should be under a new sub-heading and provide more details.
We have revised in manuscript. (line 168: In order to clarify the specific effects of metformin on microbiota, given the big safety margin, healthy volunteers were recruited for a 1 week of daily oral 500mg dose of metformin trial. Fecal samples were collected before and after oral administration of metformin for metagenomic analysis .”)
Line 197 - It's unclear why the current study conflicts with prior literature. Is it due to the disease model, the starting microbiota, something else? Please add more discussion.
Thank you for bringing this important point to our attention, and we appreciate your valuable input. We agree that it is important to discuss the potential reasons for the discrepancy between our findings and prior literature on metformin-reshaped microbiota. In our study, we used a disease model of HIRI, which may have unique characteristics compared to other disease models. It is possible that the specific disease model influenced the response of the gut microbiota. Additionally, the starting microbiota of the recipients and the characteristics of the donor microbiota used for FMT could also play a role in the disparity. We have expanded the discussion section of our revised manuscript to further address these potential factors and their implications. We hope that this additional information will provide a more comprehensive explanation for the discrepancy between our study and prior literature.
Figure 1a - change to Kaplan Meier not ANOVA. Specify the contrast - which groups are being compared?
We have revised in Figure 1a.
Figure 1e, alpha diversity - relabel "sobs" with "observed OTUs". Change to 3 bars with error and add statistics.
We have revised in Figure 1e.
Figure 1e, PCA - this should be a separate panel (1f). Color of big red circle doesn't match the points. Add PERMANOVA p-value/R2. Change to OTUs not genera. Better yet, use amplicon sequence variants from DADA2.
We have revised in Figure 1e..
Figure 2a - Change to Kaplan Meier. Also, it's unclear if residual metformin could be in the donor samples.
We have revised in Figure 2a.
Figure 2f, alpha diversity - relabel "sobs" with "observed OTUs". Change to 3 bars with error and add statistics.
We have revised in Figure 2f.
Figure 2f, PCA - this should be a separate panel (2g). Color of big orange circle doesn't match the points. Add PERMANOVA p-value/R2. Change to OTUs not genera. Better yet, use amplicon sequence variants from DADA2.
We have revised in Figure 2f.
Figure 4b - check units, shouldn't this be ng/mg (i.e. weight not volume).
We have revised in Figure 4b.
Figure 4c,d - need more explanation in the legend and Results as to what is shown here.
We have revised in Figure 4c,d.
Figure 4d - unclear why only Bacteroides are shown here or if the p-values are adjusted for multiple comparisons.
Thank you for your comment regarding Figure 4d in our manuscript. We apologize for the confusion caused. The reason why only Bacteroides is shown in Figure 4d is because we specifically wanted to investigate the changes in Bacteroides abundance following metformin treatment.
In the mouse experiments, we observed a significant increase in Bacteroides after metformin treatment. To investigate if a similar change occurs in healthy volunteers, we examined the levels of Bacteroides in fecal samples before and after oral administration of metformin. We found that the abundance of Bacteroides also increased in the human gut after metformin administration, consistent with the results from the animal experiments. Regarding the p-values, we apologize for not mentioning whether they were adjusted for multiple comparisons in the figure legend. In our revised manuscript, we have provided a clarification stating that the p-values were adjusted using the appropriate method. We appreciate your feedback and hope that this explanation clarifies the rationale behind Figure 4d. Thank you for your valuable input.
Reviewer #2 (Recommendations For The Authors):
Below I've listed several suggestions to improve the paper.
- Controls - the authors should include metformin only treated mice, FMT only treated mice, etc. Additionally, germ free mice treated with metformin and HIRI would be helpful to better implicate the gut microbiome in these beneficial effects.
Thank you for your suggestion regarding the inclusion of additional control groups in our study. We agree that including metformin only treated mice, FMT only treated mice, and germ-free mice treated with metformin and HIRI would provide valuable insights into the role of the gut microbiome in the observed beneficial effects.
Therefore, we have included metformin only treated mice, FMT only treated mice and Abx only treated mice as supplement to better assess the specific contribution to the observed effects. As results shown, there were no significance among Control, Control+Met, Control+FMT and Control+Abx groups, revealing that metformin and its treated feces, and antibiotics had no effect on liver function in normal mice (figure1).
We appreciate your input and believe that the inclusion of these additional control groups will strengthen our study and provide a more comprehensive understanding of the role of the gut microbiome in the therapeutic effects observed.
Author response image 5.
Figure1 a: Liver MDA detection; b: Serum ALT level; c: Serum AST level.
- More thorough characterization of metabolite pools. Metformin is known to influence many pathways including bile acids and lipids. These important molecules should be measures as they likely play a key role in the observed protective effect. In fact, many of the key changes displayed in Figure 3H are involved in lipid metabolism.
Thank you for your valuable feedback regarding the characterization of metabolite pools in our study. We appreciate your suggestion to measure the influence of metformin on bile acids and lipid metabolism, as they are crucial pathways that may play a significant role in the observed protective effect.
Regarding bile acids, we agree that they are important in the context of metformin’s influence on metabolic pathways. However, it is important to note that the impact of metformin on bile acids appears to be more prominent in chronic liver disease models. In our acute model, the changes in bile acids were not as significant. Instead, our results primarily indicate a close association between lipid changes and hepatic ferroptosis. Metformin significantly modulates lipid metabolism, thereby alleviating liver ferroptosis.
Additionally, we have conducted metagenomic sequencing on the gut microbiota of healthy volunteers before and after oral administration of metformin. While analyzing the data, we did not observe significant changes in key genes involved in regulating bile acid variations. This might be attributed to the healthy volunteers used in our study, where significant changes in bile acids were not induced.
We appreciate your insightful comments and suggestions, which have shed light on the importance of characterizing bile acids and lipid metabolism in our study. While the impact of bile acids may be more evident in chronic liver disease models, our findings highlight the significant influence of metformin on lipid metabolism, closely related to hepatic ferroptosis. We will take your suggestions into account for future studies to further explore the role of bile acids and their regulation by metformin.
- Imaging of lipid ROS is not quantitative. The authors should conduct more standard assays with BODIPY 581/591 C11 using cell lysates.
We appreciate your suggestion to conduct more standard assays using BODIPY 581/591 C11 with cell lysates.
We would like to clarify that we did indeed utilize assays with BODIPY 581/591 C11 to detect and measure lipid ROS in our study. The detailed description of these assays can be found in the Methods section of our paper. We followed established protocols and guidelines to ensure accurate and reliable measurements of lipid ROS levels.
We acknowledge that imaging techniques may have limitations in providing quantitative data. However, we employed BODIPY 581/591 C11 assays as a widely accepted and commonly used method to assess lipid ROS levels. This allowed us to obtain qualitative and semi-quantitative information on the changes in lipid ROS levels in response to metformin treatment.
- Liproxstatin may be a better drug choice or at the very least should be used to compare with the DFO data
Thank you for your suggestion. We have taken your advice into consideration and conducted an evaluation of Liproxstatin as a ferroptosis inhibitor. Our findings indicate that Liproxstatin significantly improves HIRI (Figure C). We believe that incorporating Liproxstatin in our research will provide valuable insights and allow for a comprehensive comparison with the DFO data.
Author response image 6.
Figure3 a: Liver MDA detection; b: Serum ALT level; c: Serum AST level; d: Liver GSH level; e: Liver Fe level.
- The rationale for how GABA was selected is not clear. I am surprised that there were not more significant metabolite changes. It might be better to show a volcano plot of heatmap of the significantly changed features.
Thank you for raising an important question regarding the rationale for selecting GABA as the focus metabolite in our study. Initially, we also had concerns about the limited number of significant metabolite changes observed. However, through our comprehensive metabolomic profiling, we identified GABA as the most significantly altered metabolite following HIRI.
It is worth noting that we specifically focused on the measurement of 22 essential amino acids in our analysis. While it is possible that changes in non-essential amino acids may have occurred, we did not examine them in this study. Nevertheless, we have since used additional methods to validate the upregulation of GABA levels, and the biological effects observed support the specific role of GABA in protecting against HIRI. Based on the fact that GABA was the only significant amino acid, the volcano plot was of little significance, so we did not supplement this plot.
We appreciate your valuable input and thank you for bringing up this important issue.
- The manuscript needs to be proofread and edited. There are a variety of typos and grammar issues throughout.
Thank you for your feedback. We acknowledge that the manuscript requires proofreading and editing, as we have identified several typos and grammar issues. We will try to ensure that the necessary revisions are made to improve the overall quality of the manuscript.
Reviewer #3 (Recommendations For The Authors):
However, I have some major concerns for the manuscript.
- Line 26 16S rRNA and metagenomic sequencing alone can't accurately confirm the improvement effect of GABA producing bacteria on HIRI. In fact, transcriptome analysis, HPLC-MS/MS and other methods were also used in this paper, so the language expression here is not appropriate
Thank you for pointing out the language expression issue in line 26 of the manuscript. We apologize for any confusion caused. You are correct in stating that 16S rRNA and metagenomic sequencing alone may not accurately confirm the improvement effect of GABA-producing bacteria on HIRI. In our study, we employed a combination of multiple methods, including transcriptome analysis, HPLC-MS/MS, especially detection of bacteria GABA key synthetases, PAT and GAD, to comprehensively investigate the impact of GABA-producing bacteria on HIRI.
We have revised the language in line 26 to reflect the broader range of methods used in our study to support the conclusions regarding the improvement effect of GABA-producing bacteria on HIRI.
- The Introduction section needs to add a description of the previous research on the association between HIRI and ferroptosis
Thank you for your suggestion regarding the inclusion of a description of the association between HIRI and ferroptosis in the Introduction section. We agree that this is an important aspect to address. However, upon further consideration, we have decided to move the discussion of ferroptosis and its potential role in HIRI to the Discussion section, as it aligns better with the logical flow of the manuscript. This allows us to discuss the potential implications and future directions in a more organized and coherent manner.
- Authors should provide quantified figure or table next to the results of western blot that are more convenient to understand.
We have revised in manuscript. (See sfigure 7)
- In this paper, FMT experiments are used to verify that metformin remodeled gut microbiota can play a role in improving HIRI. The operation steps of FMT should be described more specifically in the method part
*What is the fecal donor information for FMT?
*Line272 Did the IR + FMT group put the transplanted microbiota of FMT directly into the drinking water like the other treatment groups? Will such an operation affect the quality and quantification of the transplanted microbiota and lead to the loss of microbiota species? It is crucial for the authors to provide a clear and thorough clarification regarding these matters within the context of their FMT experiment.
Thank you for your feedback regarding the need for a more detailed description of the fecal microbiota transplantation (FMT) procedure and clarification regarding the IR + FMT group in our manuscript. We appreciate your suggestions and we have taken them into consideration.
In our study, the fecal donor for FMT was obtained from mice that had been orally administered metformin. The fecal microbiota was collected and processed to remove any residual metformin before transplantation. Specifically, the microbiota for the IR + FMT group was administered through gavage, as stated in line 272. This method does not affect the quality or quantity of the transplanted microbiota, nor does it lead to a loss of microbiota species. We understand the importance of providing clear and thorough clarification regarding these matters. Therefore, we have included additional specific details of the FMT procedure in the revised version of the manuscript. We hope that this clarification addresses your concerns and provides a more comprehensive understanding of our FMT experiment.
- The presentation of transcriptomic analysis results in the manuscript is insufficiently comprehensive and specific, as they are solely depicted through Fig 4a. Relying solely on Fig 4a is inadequate to establish the definitive roles of the met group and FMT group in ferroptosis compared to other groups. Therefore, the authors should provide additional transcriptomic analysis results to ascertain the specific effects of the met group and FMT group in ferroptosis, as well as their comparison with other groups.
Thank you for your feedback regarding the comprehensiveness of our transcriptomic analysis results in the manuscript. We understand your concerns and appreciate your suggestion. In our study, we have provided additional data beyond Fig 4a to support the specific effects of the met group and FMT group in ferroptosis, as well as their comparison with other groups. Specifically, in Figure 3, we have included Western blot (WB) and quantitative real-time polymerase chain reaction (qRT-PCR) data to confirm the involvement of ferroptosis in HIRI and the role of metformin in attenuating ferroptosis. Moreover, we have presented transcriptomic analysis results in Figure 3h, which includes a heatmap of genes related to lipid metabolism. These findings can strengthen our conclusions regarding the importance of ferroptosis in HIRI and the protective effects of metformin against ferroptosis. We hope that these data address your concerns and provide a more comprehensive understanding of our research findings.