Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorMichael DustinUniversity of Oxford, Oxford, United Kingdom
- Senior EditorTadatsugu TaniguchiThe University of Tokyo, Tokyo, Japan
Reviewer #1 (Public Review):
Summary: The authors started by stimulating the PBMCs in bulk, then encapsulated single cells in droplets to monitor the secreted cytokines in each droplet for the next 4 hours. The secreted cytokines are bound by fluorescently labeled detection antibodies. At the same time, the cytokines can be captured by the capture antibodies that are immobilized to the magnetic beads. Under the magnetic field, the magnetic beads will line up in the middle of the droplet along with bound fluorescent antibodies. This effectively enriches the fluorescent antibody to the middle of the droplet, making it a higher fluorescent signal compared to the background signal that is in the rest of the droplet. They can parallel the measurement of three cytokines in each droplet.
Strengths: Observed heterogeneous cytokine secretion dynamics, which they have reported in their previous paper as well.
Weaknesses:
Since they used PBMCs, without other assay to confirm the cell subtypes, I am not sure if any of the heterogeneity they detected in 6 cytokine secretion would be able to relate back to biology. In addition, the two panels were measured on separate cells, I am not sure it is meaningful to make any comparisons of the two panels as they are on different cells.
Their revision failed to make much improvement.
Reviewer #2 (Public Review):
The responses to the comments and changes in the manuscript are convincing, especially the secretion patterns of high and low secreting cells are interesting and reassuring. The only criticism I still have is that most observations are already published in the previous paper by the same authors.
Summary:
In their manuscript titled "Stimulation-induced cytokine polyfunctionality as a dynamic concept," the authors investigate the dynamic nature of polyfunctional cytokine responses to established stimulants. The authors use their previously published single-cell encapsulation droplet-microfluidic platform to analyse the response of peripheral blood mononuclear cells (PBMCs) to different stimulants and measure the secretion dynamics of individual cytokines. This assay shows that polyfunctionality in cytokine responses is a complex but short-lived phenomenon that decreases with prolonged stimulation times. The study finds that polyfunctional cells predominantly display elevated cytokine concentrations with similar secretion patterns but higher secretion levels compared to their monocytokine-secreting counterparts. The method is promising to analyse the correlation between the secretion dynamics of different cytokines in primary samples and heterogeneous cell populations.
Strengths:
This method provides single-cell-resolved and dynamic cytokine concentration information, which might be used to identify "fingerprints" of secretion patterns for selected cytokines. When extending the available data to more than one donor, this might be the basis for a diagnostic tool. The combination of established droplet microfluidics with an epi-fluorescence microscope-based readout makes it convincing that the method is transferable to other labs. Specifically, the dynamic analysis of cytokine concentrations is interesting, and the differences or similarities in secretion timepoints might be missed with end-point methods. The authors convincingly show that they detect up to three different cytokines in single cells.
Weaknesses:
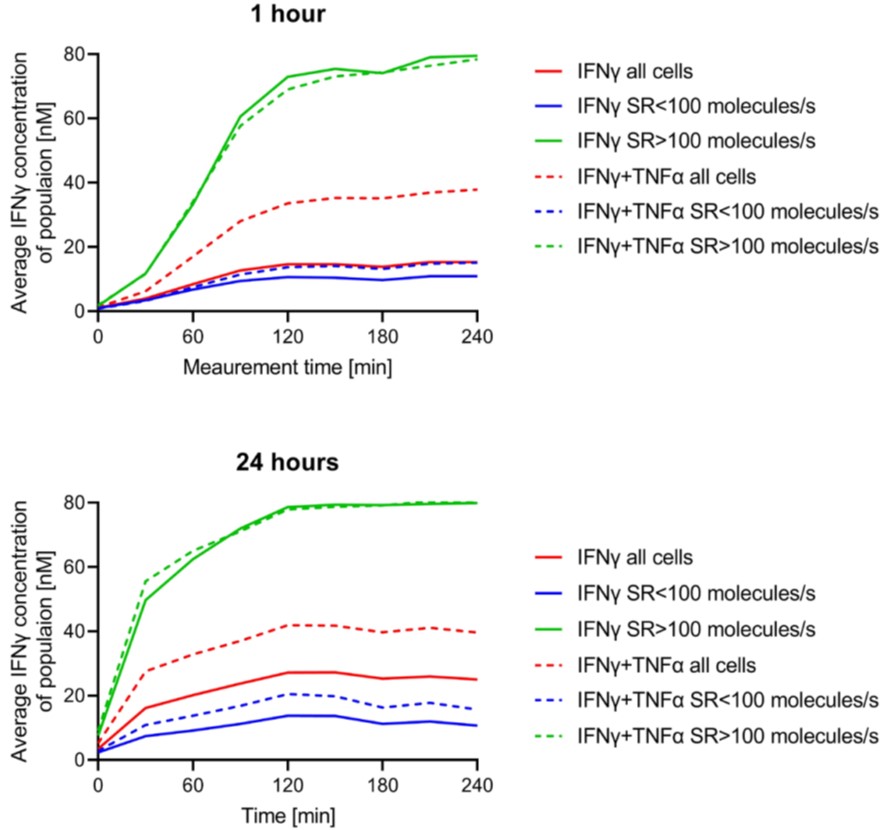
The conclusions of the study are based on samples from a single donor, which makes the conclusions on secretion patterns difficult to interpret. The choice of cytokines is explained, but the justification of the groupings of the antibodies into the two panels is missing. It would further be helpful to discuss how the single cell incubation might affect the secretion dynamics vs. the influence of co-culture of all cell types during the 24 h activation. The authors compare average secretion rates and levels. However, the right panel in Fig. 6 looks like there might be two different populations of mono- or polyfuntional cells that have two secretion rates. As the authors have single-cell data, I would find the separation into these populations more meaningful than comparing the mean values. In line with this comment, comparing the mean values for these cytokines instead of the mean of the populations with distinct secretion properties might actually show stronger differences than the authors report here.Is the plateau of the cytokine concentration caused by the fluorescence signal saturating the camera, saturation of the magnetic beads, exhaustion of the fluorescent antibodies, or constant cytokine concentrations? The high number of non-CSCs and the limited number of droplets decrease the statistical power of the method. The authors discuss their choice to use PBMCs and not solely T cells, but this aspect is missing in the discussion.