Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorWei YanWashington State University, Pullman, United States of America
- Senior EditorWei YanWashington State University, Pullman, United States of America
Reviewer #1 (Public review):
Summary:
This study generated 3D cell constructs from endometrial cell mixtures that were seeded in the Matrigel scaffold. The cell assemblies were treated with hormones to induce a "window of implantation" (WOI) state. Although many bioinformatic analyses point in this direction, there are major concerns that must be addressed.
Strengths:
The addition of 3 hormones to enhance the WOI state (although not clearly supported in comparison to the secretory state).
Comments on revisions:
The authors did their best to revise their study according to the Reviewers' comments. However, the study remains unconvincing, incomplete and at the same time still too dense and not focused enough.
Reviewer #2 (Public review):
Zhang et al. have developed an advanced three-dimensional culture system of human endometrial cells, termed a receptive endometrial assembloid, that models the uterine lining during the crucial window of implantation (WOI). During this mid-secretory phase of the menstrual cycle, the endometrium becomes receptive to an embryo, undergoing distinctive changes. In this work, endometrial cells (epithelial glands, stromal cells, and immune cells from patient samples) were grown into spheroid assembloids and treated with a sequence of hormones to mimic the natural cycle. Notably, the authors added pregnancy-related factors (such as hCG and placental lactogen) on top of estrogen and progesterone, pushing the tissue construct into a highly differentiated, receptive state. The resulting WOI assembloid closely resembles a natural receptive endometrium in both structure and function. The cultures form characteristic surface structures like pinopodes and exhibit abundant motile cilia on the epithelial cells, both known hallmarks of the mid-secretory phase. The assembloids also show signs of stromal cell decidualization and an epithelial mesenchymal transition, like process at the implantation interface, reflecting how real endometrial cells prepare for possible embryo invasion.
Although the WOI assembloid represents an important step forward, it still has limitations: the supportive stromal and immune cell populations decrease over time in culture, so only early-passage assembloids retain full complexity. Additionally, the differences between the WOI assembloid and a conventional secretory-phase organoid are more quantitative than absolute; both respond to hormones and develop secretory features, but the WOI assembloid achieves a higher degree of differentiation due to the addition of "pregnancy" signals. Overall, while it's a reinforced model (not an exact replica of the natural endometrium), it provides a valuable in vitro system for implantation studies and testing potential interventions, with opportunities to improve its long-term stability and biological fidelity in the future.
Author response:
The following is the authors’ response to the previous reviews
We have thoroughly addressed all the reviewers’ comments and meticulously revised the manuscript. Key modifications include the following:
(a) Organizing the Logic and Highlighting Key Findings: We have revised the manuscript to emphasize key findings (especially the distinctions between the SEC and WOI groups) according to the following logic: constructing a receptive endometrial organoid, comparing its molecular characteristics with those of the receptive endometrium, highlighting its main features (hormone response, enhanced energy metabolism, ciliary assembly and motility, epithelial-mesenchymal transition), and exploring the function involved in embryo interaction.
(b) Clarity and Better Description of Bioinformatic Analyses: We have revised the sections involving bioinformatic analyses to provide a more streamlined and comprehensible explanation. Instead of overwhelming the reader with excessive details, we focused on the most important findings, and performed additional experimental validation.
(c) Rationale for Gene Selection: We have clarified the rationale for selecting certain genes and pathways for inclusion in the analysis and manuscript. The associated gene expression data for all figures have been provided in the attached Dataset.
(d) In the response letter, we have provided the detailed presentation of the methodological optimization for constructing this endometrial assembloids, along with optimization and comparison of endometrial organoid culture media. Furthermore, in the Limitations section, we have explicitly stated that stromal cells and immune cells gradually diminish with increasing passage numbers. Therefore, this study primarily utilized endometrial assembloids within the first three passages for all investigations.
Below, we provide a point-by-point response to each comment, with all modifications highlighted in the revised manuscript. We respectfully hope that these revisions effectively address the concerns raised by the reviewers.
Public Reviews:
Reviewer #1 (Public Review):
This study generated 3D cell constructs from endometrial cell mixtures that were seeded in the Matrigel scaffold. The cell assemblies were treated with hormones to induce a "window of implantation" (WOI) state. The authors did their best to revise their study according to the reviewers' comments. However, the study remains unconvincing and at the same time too dense and not focused enough.
(1) The use of the term organoids is still confusing and should be avoided. Organoids are epithelial tissue-resembling structures. Hence, the multiplecell aggregates developed here are rather "coculture models" (or "assembloids"). It is still unexpected (unlikely) that these structures containing epithelial, stromal and immune cells can be robustly passaged in the epithelial growth conditions used. All other research groups developing real organoids from endometrium have shown that only the epithelial compartment remains in culture at passaging (while the stromal compartment is lost). If authors keep to their idea, they should perform scRNA-seq on both early and late (passage 6-10) "organoids". And they should provide details of culturing/passaging/plating etc that are different with other groups and might explain why they keep stromal and immune cells in their culture for such a long time. In other words, they should then in detail compare their method to the standard method of all other researchers in the field, and show the differences in survival and growth of the stromal and immune cells.
(1) We appreciate your feedback and have revised the term 'organoids' to 'assembloids'. 2)
I. Due to budget constraints, this study did not perform scRNA-seq on both early and late passages (P6-P10). Instead, immunofluorescence staining confirmed the persistence of stromal cells at passage 6 (as shown below).
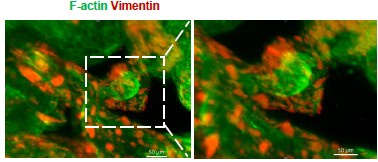
Author response image 1.
Whole-mount immunofluorescence showed that Vimentin+ F-actin+ cells (stromal cells) were arranged around the glandular spheres that were only F-actin+(passage 6).
II. Improvements in this study include the following.
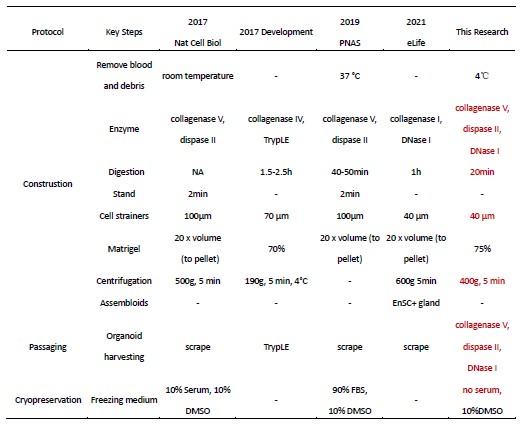
a. Optimization of endometrial tissue processing: The procedures for tissue collection, pretreatment, digestion, and culture were refined to maximize the retention of endometrial epithelial cells, stromal cells, and immune cells (detailed optimizations are provided in Response Table 1).
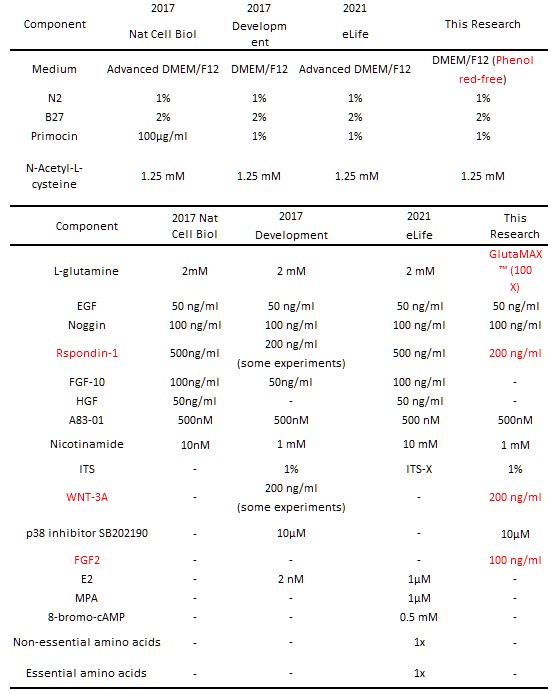
b. Enhanced culture medium formulation: Based on previous protocols, WNT3A was added to promote organoid development and differentiation (PMID: 27315476), while FGF2 was supplemented to improve stromal cell survival (PMID: 35224622) (see Response Table 2 for medium comparisons). Representative culture outcomes are shown in the figure below.
We acknowledge that the stromal and immune cells in this system still exhibit differences compared to their in vivo counterparts. In this study, we utilized the first three passages, which offer optimal cell diversity and viability, to meet experimental needs. However, replicating and maintaining the full complexity of endometrial cell types in vitro remains a major challenge in the field—one that we are actively working to address.
Author response table 1.
Methodological Optimization of Endometrial Organoids (Construction, Passaging, and Cryopreservation)
Author response table 2.
Optimization and comparison of endometrial organoid culture media
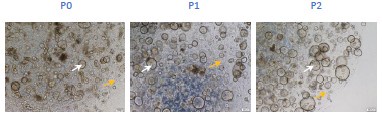
Author response image 2.
Bright-field microscopy captures the expansion of glands and surrounding stromal cells across passages 0 to 2 (scar bar=200μm) (Yellow arrows: stromal cells; White arrows: glands).
(2) The paper is still much too dense, touching upon all kind of conclusions from the manifold bioinformatic analyses. The latter should be much clearer and better described, and then some interesting findings (pathways/genes) should be highlighted without mentioning every single aspect that is observed. The paper needs a lot of editing to better focus and extract take-home messages, not bombing the reader with a mass of pathways, genes etc which makes the manuscript just not readable or 'digest-able'. There is no explanation whatever and no clear rationale why certain genes are included in a list while others are not. There is the impression that mass bioinformatics is applied without enough focus.
Thanks for your suggestions. We have made improvements and revisions in the following areas:
(a) Clarity and Better Description of Bioinformatic Analyses: We have revised the sections involving bioinformatic analyses to provide a more streamlined and comprehensible explanation. Instead of overwhelming the reader with excessive details, we focused on the most important findings.
(b) Organizing the Logic and Highlighting Key Findings: We have revised the manuscript to emphasize key findings according to the following logic: constructing a receptive endometrial organoid, comparing its molecular characteristics with those of the receptive endometrium, highlighting its main features (hormone response, enhanced energy metabolism, ciliary assembly and motility, epithelial-mesenchymal transition), and exploring the function involved in embryo interaction.
(c) Rationale for Gene Selection: We have clarified the rationale for selecting certain genes and pathways for inclusion in the analysis and manuscript.
We hope these revisions address your concerns and improve the overall quality and clarity of the manuscript. Thank you once again for your valuable input.
(3) The study is much too descriptive and does not show functional validation or exploration (except glycogen production). Some interesting findings extracted from the bioinformatics must be functionally tested.
Thanks for your suggestions. We have restructured the logic and revised the manuscript, incorporating functional validation. The focus is on the following points: highlighting its main features (hormone response, enhanced energy metabolism, ciliary assembly and motility, epithelial-mesenchymal transition), and exploring the functions involved in embryo interaction.
(4) In contrast to what was found in vivo (Wang et al. 2020), no abrupt change in gene expression pattern is mentioned here from the (early-) secretory to the WoI phase. Should be discussed. Although the bioinformatic analyses point into this direction, there are major concerns which must be solved before the study can provide the needed reliability and credibility for revision.
To further investigate the abrupt change, the Mfuzz algorithm was utilized to analyze gene expression across the three groups, focusing on gene clusters that were progressively upregulated or downregulated. It was observed that mitochondrial and cilia-related genes exhibited the highest expression levels in WOI endometrial organoids, as well as cell junction and negative regulation of cell differentiation were downregulated (Figure 4A).
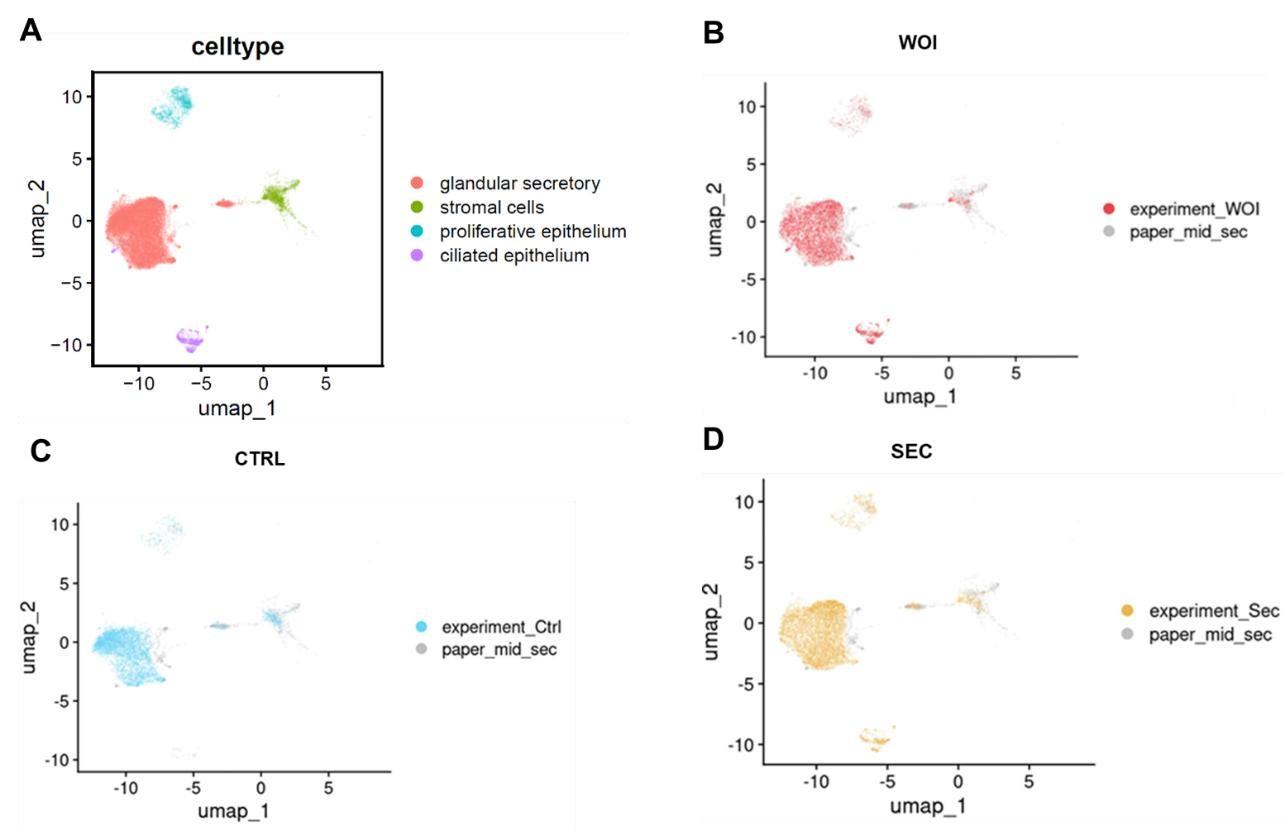
(5) All data should be benchmarked to the Wang et al 2020 and Garcia-Alonso et al. 2021 papers reporting very detailed scRNA-seq data, and not only the Stephen R. Quake 2020 paper.
We appreciate your suggestion. By integrating data from Garcia-Alonso et al. (2021) (shown in the figure below), we observed that both WOI organoids and SEC organoids exhibit increased glandular secretory epithelium and developed ciliated epithelium, mirroring features of mid-secretory endometrium. The findings exhibit parallels when contrasting these two papers.
Author response image 3.
UMAP visualization of integrated scRNA-seq data (our dataset and Garcia-Alonso et al. 2021) showing: (A) cell types, (B) WOI-org, (C)CTRL-org, (D)SEC-org versus published midsecretory samples.
(6) Fig. 2B: Vimentin staining is not at all clear. F-actin could be used to show the typical morphology of the stromal cells?
We appreciate your suggestion. We performed additional staining for F-actin based on Vimentin, and found that Vimentin+ F-actin+ cells (stromal cells) were arranged around the glandular spheres that were only F-actin+.
(7) Where does the term "EMT-derived stromal cells" come from? On what basis has this term been coined?
Within endometrial biology, stromal cells in the transition from epithelial to mesenchymal phenotype are specifically referred to as 'stromal EMT transition cells' (PMID: 39775038, PMID: 39968688).
In certain cancers or fibrotic diseases, epithelial cells can transition into a mesenchymal phenotype, contributing to the stromal environment that supports tumor growth or tissue remodeling (PMID: 20572012).
(8) CD44 is shown in Fig. 2D but the text mentions CD45 (line 159)?
In Fig 2D, T cells are defined as a cluster of CD45+CD3+ cells, further subdivided into CD4+ and CD8+ T cells based on their expression of CD4 and CD8. This figure does not include data on CD44.
(9) All quantification experiments (of stainings etc) should be in detail described how this was done. It looks very difficult (almost not feasible) when looking at the provided pictures to count the stained cells.
a. Manual Measurement:
For TEM-observed pinopodes, glycogen particles, microvilli, and cilia, manual region-of-interest (ROI) selection was performed using ImageJ software for quantitative analysis of counts, area, and length. Twenty randomly selected images per experimental group were analyzed for each morphological parameter.
b. Automated Measurement:
We quantified the fluorescence images using ImageJ. Firstly, preprocess them by adjusting brightness and contrast, and removing background noise with the “Subtract Background” feature.
Secondly, set the threshold to highlight the cells, then select the regions of interest (ROI) using selection tools. Thirdly, as for counting the cells, navigate to Analyze > Analyze Particles. AS for measuring the influence intensity and area, set the “Measurement” options as mean gray value. Adjust parameters as needed, and view results in the “Results” window. Save the data for further analysis and ensure consistency throughout your measurements for reliable results.
For 3D fluorescence quantification, ZEN software (Carl Zeiss) was exclusively used, with 11 images analyzed per experimental group. This part has been incorporated into “Supporting Information”
Line 94-100.
c. Normalization Method:
For fluorescence quantification, DAPI was used as an internal reference for normalization, where both DAPI and target fluorescence channel intensities were quantified simultaneously. The normalized target signal intensity (target/DAPI ratio) was then compared across experimental groups. A minimum of 15 images were analyzed for each parameter per group. This part has been incorporated into “Supporting Information” Line 101-104.
(10) Fig. 3C: it is unclear how quantification can be reliably done. Moreover, OLFM4 looks positive in all cells of Ctrl, but authors still see an increase?
(a) Fluorescence images were quantitatively analyzed using ImageJ by measuring the mean gray values. For normalization, DAPI staining served as an internal reference, with simultaneous measurement of mean gray values in both the target fluorescence channel and the DAPI channel. The relative fluorescence intensity was then calculated as the ratio of target channel to DAPI signal for inter-group quantitative comparisons.
(b) OLFM4 is an E2-responsive gene. Its expression in endometrial organoids of the CTRL group is physiologically normal (PMID: 31666317). However, its fluorescence intensity (quantified as mean gray value) was significantly stronger in both the SEC and WOI groups compared to the CTRL group (quantitative method as described above).
(11) Fig. 3F: Met is downregulated which is not in accordance with the mentioned activation of the PI3K-AKT pathway.
We appreciate your careful review. Our initial description was imprecise. In the revised manuscript, this statement has been removed entirely.
(12) Lines 222-226: transcriptome and proteome differences are not significant; so, how meaningful are the results then? Then, it is very hard to conclude an evolution from secretory phase to WoI.
We appreciate your feedback. The manuscript has been comprehensively revised, and the aforementioned content has been removed.
(13) WoI organoids show an increased number of cilia. However, some literature shows the opposite, i.e. less ciliated cells in the endometrial lining at WoI (to keep the embryo in place). How to reconcile?
Thank you for raising this question. We conducted a statistical analysis of the proportion of ciliated cells across endometrial phases.
(a) Based on the 2020 study by Stephen R. Quake and Carlos Simon’s team published in Nature Medicine (PMID: 32929266), the mid-secretory phase (Days 19–23) exhibited a higher proportion of ciliated cells compared to the early-secretory (Days 15–18) and late-secretory phases (Days 24– 28) (Fig. R13 A).
(b) According to the 2021 study by Roser Vento-Tormo’s team in Nature Genetics, ciliated cell abundance peaked in the early-to-mid-secretory endometrium across all phases (Fig. R13 B-C).
Data were sourced from the Reproductive Cell Atlas.
(14) How are pinopodes distinguished from microvilli? Moreover, Fig. 3 does not show the typical EM structure of cilia.
Thank you for this insightful question.
(a) Pinopodes are large, bulbous protrusions with a smooth apical membrane. Under transmission electron microscopy (TEM), it can be observed that the pinopodes contain various small particles, which are typically extracellular fluid and dissolved substances.
Microvilli are elongated, finger-like projections that typically exhibit a uniform and orderly arrangement, forming a "brush border" structure. Under transmission electron microscopy, dense components of the cytoskeleton, such as microfilaments and microtubules, can be seen at the base of the microvilli.
(b) You may refer to the ciliated TEM structure shown in the current manuscript's Fig. 2E (originally labeled as Fig. 2H in the draft). The cilium is composed of microtubules. The cross-section shows that the periphery of the cilium is surrounded by nine pairs of microtubules arranged in a ring. The longitudinal section shows that the cilium has a long cylindrical structure, with the two central microtubules being quite prominent and located at the center of the cilium.
(15) There is a recently published paper demonstrating another model for implantation. This paper should be referenced as well (Shibata et al. Science Advances, 2024).
Thanks for your valuable comments. We have cited this reference in the manuscript at Line 77-78.
(16) Line 78: two groups were the first here (Turco and Borreto) and should both be mentioned.
Thanks for your valuable comments. We have cited this reference in the manuscript at Line 74-76.
(17) Line 554: "as an alternative platform" - alternative to what? Authors answer reviewers' comments by just changing one word, but this makes the text odd.
Thank you for your review. Here, we propose that this WOI organoid serves as an alternative research platform for studying endometrial receptivity and maternal-fetal interactions, compared to current secretory-phase organoids. In the revised manuscript, we have supplemented the data by co-culturing this WOI organoid with blastoid, demonstrating its robust embryo implantation potential.
Reviewer #2 (Public Review):
In this research, Zhang et al. have pioneered the creation of an advanced organoid culture designed to emulate the intricate characteristics of endometrial tissue during the crucial Window of Implantation (WOI) phase. Their method involves the incorporation of three distinct hormones into the organoid culture, coupled with additives that replicate the dynamics of the menstrual cycle. Through a series of assays, they underscore the striking parallels between the endometrial tissue present during the WOI and their crafted organoids. Through a comparative analysis involving historical endometrial tissue data and control organoids, they establish a system that exhibits a capacity to simulate the intricate nuances of the WOI. The authors made a commendable effort to address the majority of the statements. Developing an endometrial organoid culture methodology that mimics the window of implantation is a game-changer for studying the implantation process. However, the authors should strive to enhance the results to demonstrate how different WOI organoids are from SEC organoids, ensuring whether they are worth using in implantation studies, or a proper demonstration using implantation experiments.
Thank you for your valuable suggestions. The WOI organoids differ from SEC organoids in the following aspects.
(1) Structurally, WOI endometrial organoids exhibit subcellular features characteristic of the implantation window: densely packed pinopodes on the luminal side of epithelial cells, abundant glycogen granules, elongated and tightly arranged microvilli, and increased cilia (Figure 2F).
(2) At the molecular level, WOI organoids show enlarged and functionally active mitochondria, enhanced ciliary assembly and motility, and single-cell signatures resembling mid-secretory endometrium.
Specifically, mitochondrial- and cilia-related genes/proteins are most highly expressed in WOI organoids (Figure 4A,B). TEM analysis revealed that WOI organoids have the largest average mitochondrial area (Figure 4C). Mitochondrial-related genes display an increasing trend across the three organoid groups, and WOI organoids produce more ATP and IL-8 (Figure 4D,E).
For cilia, WOI organoids upregulate genes/proteins involved in ciliary assembly, basal bodies, and motile cilia, while downregulating non-motile cilia markers (Figure 5A-C).
Single-cell analysis further confirms that WOI organoids recapitulate mid-secretory endometrial traits in mitochondrial metabolism and cell adhesion (Figure 2G).
(3) Functionally, WOI organoids demonstrate superior embryo implantation potential. Given the scarcity and ethical constraints of human embryos, we used blastoids for implantation assays (Figure 6A). These blastoids successfully grew within endometrial organoids, established interactions (Figure 6B), and exhibited normal trilineage differentiation (epiblast: OCT4; hypoblast: GATA6; trophoblast: KRT18) (Figure 6C). WOI organoids achieved significantly higher blastoid survival (66% vs. 19% in CTRL and 28% in SEC) and interaction rates (90% vs. 47% in CTRL and 53% in SEC), confirming their robust embryo-receptive capacity (Figure 6D,E).
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
In conclusion, it is needed to first meet all the concerns of the reviewers and then submit an appropriately adapted and comprehensive paper (also showing the robustness of the "organoids" and functionality of the findings) instead of this still fully descriptive paper. Further comments are included in the rebuttal document of the authors and will be provided by the editor as PDF.
Reviewer #2 (Recommendations For The Authors):
The authors made a good effort to reply all the statements. However, there are some points that the authors need to address.
• There is an inconsistency in the manuscript regarding the number of passages in which the organoids are used; in the response to the reviewers, it mentions 5 passages, while in the Materials and Methods section, it states 3 passages.
We sincerely appreciate your thorough review. In this study, organoids within the first three passages were used. To address the reviewer's question comprehensively, we have now provided a detailed account of the organoid passage history in our response.
• We agree that the difference between SEC and WOI organoids may be subtle, but in response to this, the authors should explain what they mean by "the most notable differences lie in the more comprehensive differentiation and varied cellular functions exhibited by WOI organoids..."
In the original manuscript, this statement indicated that, at the single-cell level, WOI endometrial organoids exhibited more functionally mature and thoroughly differentiated characteristics compared to SEC endometrial organoids (See details below).
In the revised version, we have restructured this section to focus on following aspects: hormone response, energy metabolism, ciliary assembly and motility, epithelial-mesenchymal transition, and embryo implantation potential. Consequently, the "the most notable differences lie in the more comprehensive differentiation and varied cellular functions exhibited by WOI organoids..."has been removed.
(1) Varied cellular functions:
a. Secretory Epithelium: Compared to SEC organoids, WOI organoids exhibit enhanced peptide metabolism and mitochondrial energy metabolism in their secretory epithelium, supporting endometrial decidualization and embryo implantation (Figure 3F).
b. Proliferative Epithelium: Compared to SEC organoids, WOI organoids demonstrate enhanced GTPase activity, angiogenesis, cytoskeletal assembly, cell differentiation, and RAS protein signaling in their proliferative epithelium (Figure S2G).
c. Ciliated Epithelium: The ciliated epithelium of WOI endometrial organoids is associated with the regulation of vascular development and exhibits higher transcriptional activity compared to SEC organoids (Figure 5E).
d. Stromal Cells: Compared to SEC organoids, WOI organoids exhibit enhanced cell junctions, cell migration, and cytoskeletal regulation in EMT-derived stromal cells (Figure S4A right panel). Similarly, cell junctions are also strengthened in stromal cells (Figure S4A left panel).
(2) comprehensive differentiation:
a. Compared to SEC organoids, WOI organoids exhibit more complete differentiation from proliferative epithelium to secretory epithelium (Figure 3G).
b. The WOI organoids demonstrate more robust ciliary differentiation compared to SEC organoids (Figure 5F).
c. The proliferative epithelium progressively differentiates into EMT-derived cells. Compared to SEC organoids, WOI organoids are predominantly localized at the terminal end of the differentiation trajectory, indicating more complete differentiation (Figure S4B).
• What do the authors mean by "average intensity" when referring to the extra reagents added to the WOI? The results that the authors show in response to Reviewer 2's Q1 must be included as part of the results and explain how it was done in the materials and methods section.
This parameter indicates the growth status of organoids. It measures the gray value of organoids through long-term live-cell tracking. When organoids undergo apoptosis, they progressively condense into denser solid spheres, leading to an increase in gray value (average intensity). This content has been incorporated into the Results section (Line 129) and is further explained in the Supporting Information "Materials and Methods" (Lines 70-77).
• In panel 1C, it is not possible to see the stromal cells around because they are brightfield images.
You are partly right. Bright-field images alone indeed make it difficult to distinguish stromal cells. However, by combining whole-mount immunofluorescence staining with the characteristic elongated spindle-shaped morphology of stromal cells, we were able to roughly determine their distribution in the bright-field images.
• Responding to Reviewer 2's question Q7, the authors indicate how they establish the cluster. However, they do not specify whether they extrapolate the data from a database or create the cluster themselves based on the literature. It should be stated from which classification list (or classification database) the extrapolation has been made.
Within endometrial biology, stromal cells in the transition from epithelial to mesenchymal phenotype are specifically referred to as 'stromal EMT transition cells' (PMID: 39775038, PMID: 39968688).
In certain cancers or fibrotic diseases, epithelial cells can transition into a mesenchymal phenotype, contributing to the stromal environment that supports tumor growth or tissue remodeling (PMID: 20572012).
• Regarding Reviewer 2's question Q8, if the authors have not been able to make comparisons with, at least, SEC organoids, unfortunately, the ERT loses much of its strength and should not serve as support.
We agree with you at this point. These results have been moved to the supplementary figures.
• If the differences in the transcriptome and proteome between SEC and WOI organoids are not significant, the result does not support the authors' model. If there are barely any differences at the proteome and transcriptome level between SEC and WOI organoids, why would anyone choose to use their model over SEC organoids?
We sincerely appreciate your valuable feedback. In this revised manuscript, we have further integrated transcriptomic and proteomic analyses, revealing that WOI organoids exhibit enlarged and functionally active mitochondria, along with enhanced cilia assembly and motility compared to SEC organoids. Additionally, using a blastoid model, we demonstrated that WOI organoids possess superior embryo implantation potential, significantly outperforming SEC organoids. Our research group aims to develop an embryo co-culture model. Through systematic comparisons of structural, molecular, and co-culture characteristics between SEC and WOI organoids, we ultimately confirmed the superior performance of WOI organoids.
• SEC and WOI organoids must be different enough to establish a new model, and the authors do not demonstrate that they are.
Thank you for your valuable feedback. In the revised manuscript, we have emphasized the distinctions between SEC and WOI organoids in terms of structure, molecular characteristics, and functionality (co-culture with blastoid), as detailed below.
(1) Structurally, WOI endometrial organoids exhibit subcellular features characteristic of the implantation window: densely packed pinopodes on the luminal side of epithelial cells, abundant glycogen granules, elongated and tightly arranged microvilli, and increased cilia (Figure 2F).
(2) At the molecular level, WOI organoids show enlarged and functionally active mitochondria, enhanced ciliary assembly and motility, and single-cell signatures resembling mid-secretory endometrium.
Specifically, mitochondrial- and cilia-related genes/proteins are most highly expressed in WOI organoids (Figure 4A,B). TEM analysis revealed that WOI organoids have the largest average mitochondrial area (Figure 4C). Mitochondrial-related genes display an increasing trend across the three organoid groups, and WOI organoids produce more ATP and IL-8 (Figure 4D,E).
For cilia, WOI organoids upregulate genes/proteins involved in ciliary assembly, basal bodies, and motile cilia, while downregulating non-motile cilia markers (Figure 5A-C).
Single-cell analysis further confirms that WOI organoids recapitulate mid-secretory endometrial traits in mitochondrial metabolism and cell adhesion (Figure 2G).
(3) Functionally, WOI organoids demonstrate superior embryo implantation potential. Given the scarcity and ethical constraints of human embryos, we used blastoids for implantation assays (Figure 6A). These blastoids successfully grew within endometrial organoids, established interactions (Figure 6B), and exhibited normal trilineage differentiation (epiblast: OCT4; hypoblast: GATA6; trophoblast: KRT18) (Figure 6C). WOI organoids achieved significantly higher blastoid survival (66% vs. 19% in CTRL and 28% in SEC) and interaction rates (90% vs. 47% in CTRL and 53% in SEC), confirming their robust embryo-receptive capacity (Figure 6D,E).
• Regarding Q16, Boretto et al. 2017 and Turco et al. 2017 also manage to isolate stromal cells, but they lose them between passages. It's not a matter of isolating them from the tissue or not, but rather how they justify their maintenance in culture. In the images added by the authors, it can be seen that the majority of stromal cells are lost from P0 to P1 after thawing. I still believe that the epithelial part can be passed and maintained, but the rest cannot, and that should be mentioned in the paper as a limitation. However, the authors can demonstrate the maintenance of stromal cells by performing immunostaining with vimentin from passages 4, 5, and 6.
Thank you for your valuable comments. We have added the statement 'Stromal cells and immune cells are difficult to pass down stably and their proportion is lower than that in the in vivo endometrium' to the Limitations section (Line 364-365). Additionally, we performed immunostaining with vimentin starting from passage 6 and confirmed the presence of Vimentin+ F-actin+ stromal cells (as shown in Author response image 1).