Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorAlphee MichelotMechanobiology Institute, Singapore, Singapore
- Senior EditorMerritt MadukeStanford University, Stanford, United States of America
Reviewer #3 (Public Review):
Summary:
In this work, the authors plate different type of cells on circular micropatterns and question how the organization and dynamics of the actin cytoskeleton correlate with particular actin chiral properties and rotational direction of the nucleus. The observe that cell spreading on large patterns correlates with the emergence of anti-clockwise rotations (ACW), while spreading on small patterns leads preferentially to clockwise rotations (CW). ACW originate, as previously demonstrated, from the polymerization of radial fibers, while clockwise rotations (CW) are observed when radial fibers are disorganized or absent and when transverse arcs take over to power CW rotations. These data are supported by a large number of observations and use of multiple drugs lead to observations that are consistent with the proposed model.
Strengths:
This is a beautiful work in which the authors rely on a large number of high-quality microscopic observations and use a full arsenal of drugs to test their model as thoroughly as possible.
This study examines the influence of multiple actin networks. This is a challenging task in that the assembly and dynamics of different actin networks are interdependent, making it difficult to unambiguously analyze the importance of any specific network.
Author response:
The following is the authors’ response to the original reviews.
Preliminary note from the Reviewing Editor:
The evaluations of the two Reviewers are provided for your information. As you can see, their opinions are very different.
Reviewer #1 is very harsh in his/her evaluation. Clearly, we don't expect you to be able to affect one type of actin network without affecting the other, but rather to change the balance between the two. However, he/she also raises some valid points, in particular that more rationale should be added for the perturbations (also mentioned by Reviewer #2). Both Reviewers have also excellent suggestions for improving the presentation of the data.
We sincerely appreciate your and the reviewers’ suggestions. The comments are amended accordingly.
On another point, I was surprised when reading your manuscript that a molecular description of chirality change in cells is presented as a completely new one. Alexander Bershadsky's group has identified several factors (including alpha-actinin) as important regulators of the direction of chirality. The articles are cited, but these important results are not specifically mentioned. Highlighting them would not call into question the importance of your work, but might even provide additional arguments for your model.
We appreciate the editor’s comment. Alexander Bershadsky's group has done marvelous work in cell chirality. They introduced the stair-stepping and screw theory, which suggested how radial fiber polymerization generates ACW force and drives the actin cytoskeleton into the ACW pattern. Moreover, they have identified chiral regulators like alpha-actinin 1, mDia1, capZB, and profilin 1, which can reverse or neutralize the chiral expression.
It is worth noting that Bershadsky's group primarily focuses on radial fibers. In our manuscript, instead, we primarily focused on the contractile unit in the transverse arcs and CW chirality in our investigation. Our manuscript incorporates our findings in the transverse arcs and the radial fibers theory by Bershadsky's group into the chirality balance hypothesis, providing a more comprehensive understanding of the chirality expression.
We have included relevant articles from Alexander Bershadsky's group, we agree that highlighting these important results of chiral regulators would further strengthen our manuscript. The manuscript was revised as follows:
“ACW chirality can be explained by the right-handed axial spinning of radial fibers during polymerization, i.e. ‘stair-stepping' mode proposed by Tee et al. (Tee et al. 2015) (Figure 8A; Video 4). As actin filament is formed in a right-handed double helix, it possesses an intrinsic chiral nature. During the polymerization of radial fiber, the barbed end capped by formin at focal adhesion was found to recruit new actin monomers to the filament. The tethering by formin during the recruitment of actin monomers contributes to the right-handed tilting of radial fibers, leading to ACW rotation. Supporting this model, Jalal et al. (Jalal et al. 2019) showed that the silencing of mDia1, capZB, and profilin 1 would abolish the ACW chiral expression or reverse the chirality into CW direction. Specifically, the silencing of mDia1, capZB or profilin-1 would attenuate the recruitment of actin monomer into the radial fiber, with mDia1 acting as the nucleator of actin filament (Tsuji et al. 2002), CapZB promoting actin polymerization as capping protein (Mukherjee et al. 2016), and profilin-1 facilitating ATP-bound G-actin to the barbed ends(Haarer and Brown 1990; Witke 2004). The silencing resulted in a decrease in the elongation velocity of radial fiber, driving the cell into neutral or CW chirality. These results support that our findings that reduction of radial fiber elongation can invert the balance of chirality expression, changing the ACW-expressing cell into a neutral or CW-expressing cell.”
By incorporating their findings into our revision and discussion, we provide additional support for our radial fiber-transverse arc balance model for chirality expression. The revision is made on pages 8 to 9, 13, lines 253 to 256, 284, 312 to 313, 443, 449 to 459.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
Kwong et al. present evidence that two actin-filament based cytoskeletal structures regulate the clockwise and anticlockwise rotation of the cytoplasm. These claims are based on experiments using cells plated on micropatterned substrates (circles). Previous reports have shown that the actomyosin network that forms on the dorsal surface of a cell plated on a circle drives a rotational or swirling pattern of movement in the cytoplasm. This actin network is composed of a combination of non-contractile radial stress fibers (AKA dorsal stress fibers) which are mechanically coupled to contractile transverse actin arcs (AKA actin arcs). The authors claim that directionality of the rotation of the cytoplasm (i.e., clockwise or anticlockwise) depends on either the actin arcs or radial fibers, respectively. While this would interesting, the authors are not able to remove either actin-based network without effecting the other. This is not surprising, as it is likely that the radial fibers require the arcs to elongate them, and the arcs require the radial fibers to stop them from collapsing. As such, it is difficult to make simple interpretations such as the clockwise bias is driven by the arcs and anticlockwise bias is driven by the radial fibers.
Weaknesses:
(1) There are also multiple problems with how the data is displayed and interpreted. First, it is difficult to compare the experimental data with the controls as the authors do not include control images in several of the figures. For example, Figure 6 has images showing myosin IIA distribution, but Figure 5 has the control image. Each figure needs to show controls. Otherwise, it will be difficult for the reader to understand the differences in localization of the proteins shown. This could be accomplished by either adding different control examples or by combining figures.
We appreciate the reviewer’s comment. We agree with the reviewer that it is difficult to compare our results in the current arrangement. The controls are included in the new Figure 6.
(2) It is important that the authors should label the range of gray values of the heat maps shown. It is difficult to know how these maps were created. I could not find a description in the methods, nor have previous papers laid out a standardized way of doing it. As such, the reader needs some indication as to whether the maps showing different cells were created the same and show the same range of gray levels. In general, heat maps showing the same protein should have identical gray levels. The authors already show color bars next to the heat maps indicating the range of colors used. It should be a simple fix to label the minimum (blue on the color bar) and the maximum (red on the color bar) gray levels on these color bars. The profiles of actin shown in Figure 3 and Figure 3- figure supplement 3 were useful for interpretating the distribution of actin filaments. Why did not the authors show the same for the myosin IIa distributions?
We appreciate the reviewer’s comment. For generating the distribution heatmap, the images were taken under the same setting (e.g., fluorescent staining procedure, excitation intensity, or exposure time). The prerequisite of cells for image stacking was that they had to be fully spread on either 2500 µm2 or 750 µm2 circular patterns. Then, the location for image stacking was determined by identifying the center of each cell spread in a perfect circle. Finally, the images were aligned at the cell center to calculate the averaged intensity to show the distribution heatmap on the circular pattern. Revision is made on pages 19 to 20, lines 668 to 677.
It is important to note that the individual heatmaps represent the normalized distribution generated using unique color intensity ranges. This approach was chosen to emphasize the proportional distribution of protein within cells and its variations among samples, especially for samples with generally lower expression levels. Additionally, a differential heatmap with its own range was employed to demonstrate the normalized differences compared to the control sample. Furthermore, to provide additional insight, we plotted the intensity profile of the same protein with the same size for comparative analysis. Revision is made on pages 20, lines 679 to 682.
The labels of the heatmap are included to show the intensity in the revised Figure 3, Figure 5, Figure 6, and Figure 3 —figure supplement 4.
To better illustrate the myosin IIa distribution, the myosin intensity profiles were plotted for Y27 treatment and gene silencing. The figures are included as Figure 5—figure supplement 2 and Figure 6—figure supplement 2. Revisions are made on pages 10, lines 332 to 334 and pages 11, lines 377 to 379.
(3) Line 189 "This absence of radial fibers is unexpected". The authors should clarify what they mean by this statement. The claim that the cell in Figure 3B has reduced radial stress fiber is not supported by the data shown. Every actin structure in this cell is reduced compared to the cell on the larger micropattern in Figure 3A. It is unclear if the radial stress fibers are reduced more than the arcs. Are the authors referring to radial fiber elongation?
We appreciate the reviewer’s comment. We calculated the structures' pixel number and the percentage in the image to better illustrate the reduction of radial fiber or transverse arc. As radial fibers emerge from the cell boundary and point towards the cell center and the transverse arcs are parallel to the cell edge, the actin filament can be identified by their angle with respect to the cell center. We found that the pixel number of radial fiber is greatly reduced by 91.98 % on 750 µm2 compared to the 2500 µm2 pattern, while the pixel number of transverse arc is reduced by 70.58 % (Figure 3- figure supplement 3A). Additionally, we compared the percentage of actin structures on different pattern sizes (Figure 3- figure supplement 3B). On 2500 µm2 pattern, the percentage of radial fiber in the actin structure is 61.76 ± 2.77 %, but it only accounts for 31.13 ± 2.76 % while on 750 µm2 pattern. These results provide evidence of the structural reduction on a smaller pattern.
Regarding the radial fiber elongation, we only discussed the reduction of radial fiber on 750 µm2 compared to the 2500 µm2 pattern in this part. For more understanding of the radial fiber contribution to chirality, we compared the radial fiber elongation rate in the LatA treatment and control on 2500 µm2 pattern (Figure 4). This result suggests the potential role of radial fiber in cell chirality. Revisions are made on page 6, lines 186 to 194; pages 17 to 18, 601 to 606; and the new Figure 3- figure supplement 3.
(4) The choice of the small molecule inhibitors used in this study is difficult to understand, and their results are also confusing. For example, sequestering G actin with Latrunculin A is a complicated experiment. The authors use a relatively low concentration (50 nM) and show that actin filament-based structures are reduced and there are more in the center of the cell than in controls (Figure 3E). What was the logic of choosing this concentration?
We appreciate the reviewer’s comment. The concentration of drugs was selected based on literatures and their known effects on actin arrangement or chiral expression.
For example, Latrunculin A was used at 50 nM concentration, which has been proven effective in reversing the chirality at or below 50 nM (Bao et al., 2020; Chin et al., 2018; Kwong et al., 2019; Wan et al., 2011). Similarly, the 2 µM A23187 treatment concentration was selected to initiate the actin remodeling (Shao et al., 2015). Furthermore, NSC23677 at 100 µM was found to efficiently inhibit the Rac1 activation and resulted in a distinct change in actin structure (Chen et al., 2011; Gao et al., 2004), enhancing ACW chiral expression. The revision is made on pages 6 to 7, lines 202 to 211.
(5) Using a small molecule that binds the barbed end (e.g., cytochalasin) could conceivably be used to selectively remove longer actin filaments, which the radial fibers have compared to the lamellipodia and the transverse arcs. The authors should articulate how the actin cytoskeleton is being changed by latruculin treatment and the impact on chirality. Is it just that the radial stress fibers are not elongating? There seems to be more radial stress fibers than in controls, rather than an absence of radial stress fibers.
We appreciate the reviewer’s comment. Our results showed Latrunculin A treatment reversed the cell chirality. To compare the amount of radial fiber and transverse arc, we calculated the structures' pixel percentage. We found that, the percentage of radial fibers pixel with LatA treatment was reduced compared to that of the control, while the percentage of transverse arcs pixel increased (Figure 3— figure supplement 5). This result suggests that radial fibers are inhibited under Latrunculin A treatment.
Furthermore, the elongation rate of radial fibers is reduced by Latrunculin A treatment (Figure 4). This result, along with the reduction of radial fiber percentage under Latrunculin A treatment suggests the significant impact of radial fiber on the ACW chirality. Revisions are made on pages 7 to 8, lines 244 to 250 and the new Figure 3— figure supplement 5 and Figure 3— figure supplement 6.
(6) Similar problems arise from the other small molecules as well. LPA has more effects than simply activating RhoA. Additionally, many of the quantifiable effects of LPA treatment are apparent only after the cells are serum starved, which does not seem to be the case here.
We appreciate the reviewer’s comment. The reviewer mentioned that the quantifiable effects of LPA treatments were seen after the cells were serum-starved. LPA is known to be a serum component and has an affinity to albumin in serum (Moolenaar, 1995). Serum starvation is often employed to better observe the effects of LPA by comparing conditions with and without LPA. We agree with the reviewer that the effect of LPA cannot be fully seen under the current setting. Based on the reviewer’s comment and after careful consideration, we have decided to remove the data related to LPA from our manuscript. Revisions are made on pages 6 to 7, 17 and Figure 3— figure supplement 4.
(7) Furthermore, inhibiting ROCK with, Y-27632, effects myosin light chain phosphorylation and is not specific to myosin IIA. Are the two other myosin II paralogs expressed in these cells (myosin IIB and myosin IIC)? If so, the authors’ statements about this experiment should refer to myosin II not myosin IIa.
We appreciate the reviewer’s comment. We agree that ensuring accuracy and clarity in our statements is important. The terminology is revised to myosin II regarding the Y27632 experiment for a more concise description. Revision is made on pages 9 to 10 and 29, lines 317 to 341, 845 and 848.
(8) None of the uses of the small molecules above have supporting data using a different experimental method. For example, backing up the LPA experiment by perturbing RhoA tho.
We appreciate the reviewer’s comment. After careful consideration, we have decided to remove the data related to LPA from our manuscript. Revisions are made on pages 6 to 7, 17 and Figure 3— figure supplement 4.
(9) The use of SMIFH2 as a "formin inhibitor" is also problematic. SMIFH2 also inhibits myosin II contractility, making interpreting its effects on cells difficult to impossible. The authors present data of mDia2 knockdown, which would be a good control for this SMIFH2.
We appreciate the reviewer’s comment. We agree that there is potential interference of SMIFH2 with myosin II contractility, which could introduce confounding factors to the results. Based on your comment and further consideration, we have decided to remove the data related to SMIFH2 from our manuscript. Revisions are made on pages 6 to 7, 10, 17 and Figure 3— figure supplement 4.
(10) However, the authors claim that mDia2 "typically nucleates tropomyosin-decorated actin filaments, which recruit myosin II and anneal endwise with α-actinin- crosslinked actin filaments."
There is no reference to this statement and the authors own data shows that both arcs and radial fibers are reduced by mDia2 knockdown. Overall, the formin data does not support the conclusions the authors report.
We appreciate the reviewer’s comment. We apologize for the lack of citation for this claim. To address this, we have added a reference to support this claim in the revised manuscript (Tojkander et al., 2011). Revision is made on page 10, line 345 to 347.
Regarding the actin structure of mDia2 gene silencing, our results showed that myosin II was disassociated from the actin filament compared to the control. At the same time, there is no considerable differences in the actin structure of radial fibers and transverse arcs between the mDia2 gene silencing and the control.
(11) The data in Figure 7 does not support the conclusion that myosin IIa is exclusively on top of the cell. There are clear ventral stress fibers in A (actin) that have myosin IIa localization. The authors simply chose to not draw a line over them to create a height profile.
We appreciate the reviewer’s comment. To better illustrate myosin IIa distribution in a cell, we have included a video showing the myosin IIa staining from the base to the top of the cell (Video 7). At the cell base, the intensity of myosin IIa is relatively low at the center. However, when the focal plane elevates, we can clearly see the myosin II localizes near the top of the cell (Figure 7B and Video 7). Revision is made on page 12, lines 421 to 424, and the new Video 7.
Reviewer #2 (Public Review):
Summary:
Chirality of cells, organs, and organisms can stem from the chiral asymmetry of proteins and polymers at a much smaller lengthscale. The intrinsic chirality of actin filaments (F-actin) is implicated in the chiral arrangement and movement of cellular structures including F-actin-based bundles and the nucleus. It is unknown how opposite chiralities can be observed when the chirality of F-actin is invariant. Kwong, Chen, and co-authors explored this problem by studying chiral cell-scale structures in adherent mammalian cultured cells. They controlled the size of adhesive patches, and examined chirality at different timepoints. They made various molecular perturbations and used several quantitative assays. They showed that forces exerted by antiparallel actomyosin bundles on parallel radial bundles are responsible for the chirality of the actomyosin network at the cell scale.
Strengths:
Whereas previously, most effort has been put into understanding radial bundles, this study makes an important distinction that transverse or circumferential bundles are made of antiparallel actomyosin arrays. A minor point that was nice for the paper to make is that between the co-existing chirality of nuclear rotation and radial bundle tilt, it is the F-actin driving nuclear rotation and not the other way around. The paper is clearly written.
Weaknesses:
The paper could benefit from grammatical editing. Once the following Major and Minor points are addressed, which may not require any further experimentation and does not entail additional conditions, this manuscript would be appropriate for publication in eLife.
Recommendations for the authors:
Reviewer #2 (Recommendations For The Authors):
Major:
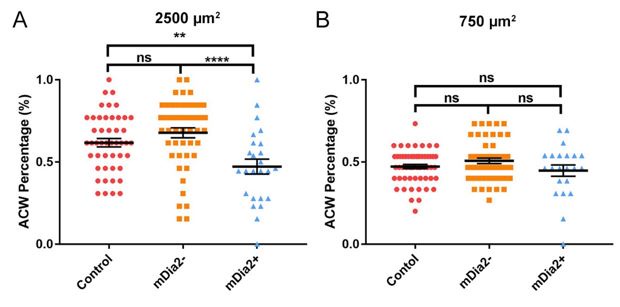
(1) The binary classification of cells as exhibiting clockwise or anticlockwise F-actin structures does not capture the instances where there is very little chirality, such as in the mDia2-depleted cells on small patches (Figure 6B). Such reports of cell chirality throughout the cell population need to be reported as the average angle of F-actin structures on a per cell basis as a rose plot or scatter plot of angle. These changes to cell-scoring and data display will be important to discern between conditions where chirality is random (50% CW, 50% ACW) from conditions where chirality is low (radial bundles are radial and transverse arcs are circumferential).
We appreciate the reviewer’s comment. We apologize if we did not convey our analysis method clearly enough. Throughout the manuscript, unless mentioned otherwise, the chirality analysis was based on the chiral nucleus rotation within a period of observation. The only exception is the F-actin structure chirality, in Figure 3—figure supplement 1, which we analyzed the angle of radial fiber of the control cell on 2500 µm2. It was described on pages 5 to 6, lines 169-172, and the method section “Analysis of fiber orientation and actin structure on circular pattern” on page 17.
Based on the feedback, we attempted to use a scatter plot to present the mDia2 overexpression and silencing to show the randomness of the result. However, because scatter plots primarily focus on visualizing the distribution, they become cluttered and visually overwhelming, as shown below.
Author response image 1.
(A) Percentage of ACW nucleus rotational bias on 2500 µm2 with untreated control (reused data from Figure 3D, n = 57), mDia2 silencing (n = 48), and overexpression (n = 25). (B) Probability of ACW/CW rotation on 750 µm2 pattern with untreated control (reused data from Figure 3E, n = 34), mDia2 silencing (n = 53), and overexpressing (n = 22). Mean ± SEM. Two-sample equal variance two-tailed t-test.
Therefore, in our manuscript, the presentation primarily used a column bar chart with statistical analysis, the Student T-test. The column bar chart makes it easier to understand and compare values. In brief, the Student T-test is commonly used to evaluate whether the means between the two groups are significantly different, assuming equal variance. As such, the Student T-test is able to discern the randomness of the chirality.
(2) The authors need to discuss the likely nucleator of F-actin in the radial bundles, since it is apparently not mDia2 in these cells.
We appreciate the reviewer’s comment. In our manuscript, we originally focused on mDia2 and Tpm4 as they are the transverse arc nucleator and the mediator of myosin II motion. However, we agree with the reviewer that discussing the radial fiber nucleator would provide more insight into radial fiber polymerization in ACW chirality and improve the completeness of the story.
Radial fiber polymerizes at the focal adhesion. Serval proteins are involved in actin nucleation or stress fiber formation at the focal adhesion, such as Arp2/3 complex (Serrels et al., 2007), Ena/VASP (Applewhite et al., 2007; Gateva et al., 2014), and formins (Dettenhofer et al., 2008; Sahasrabudhe et al., 2016; Tsuji et al., 2002), etc. Within the formin family, mDia1 is the likely nucleator of F-actin in the radial bundle. The presence of mDia1 facilitates the elongation of actin bundles at focal adhesion (Hotulainen and Lappalainen, 2006). Studies by Jalal, et al (2019) (Jalal et al., 2019) and Tee, et al (2023) (Tee et al., 2023), have demonstrated the silencing of mDia1 abolished the ACW actin expression. Silencing of other nucleation proteins like Arp2/3 complex or Ena/VASP would only reduce the ACW actin expression without abolishing it.
Based on these findings, the attenuation of radial fiber elongation would abolish the ACW chiral expression, providing more support for our model in explaining chirality expression.
This part is incorporated into the Discussion. The revision is made on page 13, lines 443, 449 to 459.
Minor:
(1) In the introduction, additional observations of handedness reversal need to be referenced (line 79), including Schonegg, Hyman, and Wood 2014 and Zaatri, Perry, and Maddox 2021.
We appreciate the reviewer’s comment. The observations of handedness reversal references are cited on page 3, line 78 to 79.
(2) For clarity of logic, the authors should share the rationale for choosing, and results from administering, the collection of compounds as presented in Figure 3 one at a time instead of as a list.
We appreciate the reviewer’s comment. The concentration of drugs was determined based on existing literature and their known outcomes on actin arrangement or chiral expression.
To elucidate, the use of Latrunculin A was based on previous studies, which have demonstrated to reverse the chirality at or below 50 nM (Bao et al., 2020; Chin et al., 2018; Kwong et al., 2019; Wan et al., 2011). Because inhibiting F-actin assembly can lead to the expression of CW chirality, we hypothesized that the opposite treatment might enhance ACW chirality. Therefore, we chose A23187 treatment with 2 µM concentration as it could initiate the actin remodeling and stress fiber formation (Shao et al., 2015).
Furthermore, in the attempt to replicate the reversal of chirality by inhibiting F-actin assembly through other pathways, we explored NSC23677 at 100 µM, which was found to inhibit the Rac1 activation (Chen et al., 2011; Gao et al., 2004) and reduce cortical F-actin assembly (Head et al., 2003). However, it failed to reverse the chirality but enhanced the ACW chirality of the cell.
We carefully selected the drugs and the applied concentration to investigate various pathways and mechanisms that influence actin arrangement and might affect the chiral expression. We believe that this clarification strengthens the rationale behind our choice of drug. The revision is made on pages 6 to 7, lines 202 to 211.
(3) "Image stacking" isn't a common term to this referee. Its first appearance in the main text (line 183) should be accompanied with a call-out to the Methods section. The authors could consider referring to this approach more directly. Related issue: Image stacking fails to report the prominent enrichment of F-actin at the very cell periphery (see Figure 3 A and F) except for with images of cells on small islands (Figure 3H). Since this data display approach seems to be adding the intensity from all images together, and since cells on circular adhesive patches are relatively radially symmetric, it is unclear how to align cells, but perhaps cells could be aligned based on a slight asymmetry such as the peripheral location with highest F-actin intensity or the apparent location of the centrosome.
We appreciate the reviewer’s comment. We fully acknowledge the uncommon use of “image stacking” and the insufficient description of image stacking under the Method section. First, we have added a call-out to the Methods section at its first appearance (Page 6, Lines 182 to 183). The method of image stacking is as follows. During generating the distribution heatmap, the images were taken under the same setting (e.g., staining procedure, fluorescent intensity, exposure time, etc.). The prerequisite of cells to be included in image stacking was that they had to be fully spread on either 2500 µm2 or 750 µm2 circular patterns. Then, the consistent position for image stacking could be found by identifying the center of each cell spreading in a perfect circle. Finally, the images were aligned at the center to calculate the averaged intensity to show the distribution heatmap on the circular pattern.
We agree with the reviewer that our image alignment and stacking are based on cells that are radially symmetric. As such, the intensity distribution of stacked image is to compare the difference of F-actin along the radial direction. Revision is made on page 19, lines 668 to 682.
(4) The authors need to be consistent with wording about chirality, avoiding "right" and left (e.g. lines 245-6) since if the cell periphery were oriented differently in the cropped view, the tilt would be a different direction side-to-side but the same chirality. This section is confusing since the peripheral radial bundles are quite radial, and the inner ones are pointing from upper left to lower right, pointing (to the right) more downward over time, rather than more right-ward, in the cropped images.
We appreciate the reviewer’s comment. We apologize for the confusion caused by our description of the tilting direction. For consistency in our later description, we mention the “right” or “left” direction of the radial fibers referencing to the elongation of the radial fiber, which then brings the “rightward tilting” toward the ACW rotation of the chiral pattern. To maintain the word “rightward tilting”, we added the description to ensure accurate communication in our writing. We also rearrange the image in the new Figure 4A and Video 2 for better observation. Revision is made on page 8, lines 262 to 263.
(5) Why are the cells Figure 4A dominated by radial (and more-central, tilting fibers, while control cells in 4D show robust circumferential transverse arcs? Have these cells been plated for different amounts of time or is a different optical section shown?
We appreciate the reviewer’s comment. The cells in Figure 4A and Figure 4D are prepared with similar conditions, such as incubation time and optical setting. Actin organization is a dynamic process, and cells can exhibit varied actin arrangements, transitioning between different forms such as circular, radial, chordal, chiral, or linear patterns, as they spread on a circular island (Tee et al., 2015). In Figure 4A, the actin is arranged in a chiral pattern, whereas in Figure 4D, the actin exhibits a radial pattern. These variations reflect the natural dynamics of actin organization within cells during the imaging process.
(6) All single-color images (such as Fig 5 F-actin) need to be black-on-white, since it is far more difficult to see F-actin morphology with red on black.
We appreciate the reviewer’s comment. We have changed all F-actin images (single color) into black and white for better image clarity. Revisions are made in the new Figure 5, Figure 6 and Figure 7.
(7) Figure 5A, especially the F-actin staining, is quite a bit blurrier than other micrographs. These images should be replaced with images of comparable quality to those shown throughout.
We appreciate the reviewer’s comment. We agree that the F-actin staining in Figure 5 is difficult to observe. To improve image clarity, the F-actin staining images are replaced with more zoomed-in image. Revision is made in the new Figure 5.
(8) F-actin does not look unchanged by Y27632 treatment, as the authors state in line 306. This may be partially due to image quality and the ambiguities of communicating with the blue-to-red colormap. Similarly, I don't agree that mDia2 depletion did not change F-actin distribution (line 330) as cells in that condition had a prominent peripheral ring of F-actin missing from cells in other conditions.
We appreciate the reviewer’s comment. We agree with the reviewer’s observation that the F-actin distribution is indeed changed under Y27632 treatment compared to the control in Figure 5A-B. Here, we would like to emphasize that the actin ring persists despite the actin structure being altered under the Y27632 treatment. The actin ring refers to the darker red circle in the distribution heatmap. It presents the condensed actin structure, including radial fibers and transverse arcs. This important structure remains unaffected despite the disruption of myosin II, the key component in radial fiber.
Furthermore, we agree with the reviewer that mDia2 depletion does change F-actin distribution. Similar to the Y27632 treatment, the actin ring persists despite the actin structure being altered under mDia2 gene silencing. Moreover, compared to other treatments, mDia2 depletion has less significant impact on actin distribution. To address these points more comprehensively, we have made revision in Y27632 treatment and mDia2 sections. The revisions of Y27632 and mDia2 are made on pages 10, lines 324-327 and 352-353, respectively.
(9) The colormap shown for intensity coding should be reconsidered, as dark red is harder to see than the yellow that is sub-maximal. Verdis is a colormap ranging from cooler and darker blue, through green, to warmer and lighter yellow as the maximum. Other options likely exist as well.
We appreciate the reviewer’s comment. We carefully considered the reviewer’s concern and explored other color scale choices in the colormap function in Matlab. After evaluating different options, including “Verdis” color scale, we found that “jet” provides a wide range of colors, allowing the effective visual presentation of intensity variation in our data. The use of ‘jet’ allows us to appropriately visualize the actin ring distribution, which represented in red or dark re. While we understand that dark red could be harder to see than the sub-maximal yellow, we believe that “jet” serves our purpose of presenting the intensity information.
(10) For Figure 6, why doesn't average distribution of NMMIIa look like the example with high at periphery, low inside periphery, moderate throughout lamella, low perinuclear, and high central?
We appreciate the reviewer’s comment. We understand that the reviewer’s concern about the average distribution of NMMIIa not appearing as the same as the example. The chosen image is the best representation of the NMMIIa disruption from the transverse arcs after the mDia2 silencing. Additionally, it is important to note that the average distribution result is a stacked image which includes other images. As such, the NMMIIA example and the distribution heatmap might not necessarily appear identical.
(11) In 2015, Tee, Bershadsky and colleagues demonstrated that transverse bundles are dorsal to radial bundles, using correlative light and electron microscopy. While it is important for Kwong and colleagues to show that this is true in their cells, they should reference Tee et al. in the rationale section of text pertaining to Figure 7.
We appreciate the reviewer’s comment. Tee, et al (Tee et al., 2015) demonstrated the transverse fiber is at the same height as the radial fiber based on the correlative light and electron microscopy. Here, using the position of myosin IIa, a transverse arc component, our results show the dorsal positioning of transverse arcs with connection to the extension of radial fibers (Figure 7C), which is consistent with their findings. It is included in our manuscript, page 12, lines 421 to 424, and page 14 lines 477 to 480.
Reference
Applewhite, D.A., Barzik, M., Kojima, S.-i., Svitkina, T.M., Gertler, F.B., and Borisy, G.G. (2007). Ena/Vasp Proteins Have an Anti-Capping Independent Function in Filopodia Formation. Mol. Biol. Cell. 18, 2579-2591. DOI: https://doi.org/10.1091/mbc.e06-11-0990
Bao, Y., Wu, S., Chu, L.T., Kwong, H.K., Hartanto, H., Huang, Y., Lam, M.L., Lam, R.H., and Chen, T.H. (2020). Early Committed Clockwise Cell Chirality Upregulates Adipogenic Differentiation of Mesenchymal Stem Cells. Adv. Biosyst. 4, 2000161. DOI: https://doi.org/10.1002/adbi.202000161
Chen, Q.-Y., Xu, L.-Q., Jiao, D.-M., Yao, Q.-H., Wang, Y.-Y., Hu, H.-Z., Wu, Y.-Q., Song, J., Yan, J., and Wu, L.-J. (2011). Silencing of Rac1 Modifies Lung Cancer Cell Migration, Invasion and Actin Cytoskeleton Rearrangements and Enhances Chemosensitivity to Antitumor Drugs. Int. J. Mol. Med. 28, 769-776. DOI: https://doi.org/10.3892/ijmm.2011.775
Chin, A.S., Worley, K.E., Ray, P., Kaur, G., Fan, J., and Wan, L.Q. (2018). Epithelial Cell Chirality Revealed by Three-Dimensional Spontaneous Rotation. Proc. Natl. Acad. Sci. U.S.A. 115, 12188-12193. DOI: https://doi.org/10.1073/pnas.1805932115
Dettenhofer, M., Zhou, F., and Leder, P. (2008). Formin 1-Isoform IV Deficient Cells Exhibit Defects in Cell Spreading and Focal Adhesion Formation. PLoS One 3, e2497. DOI: https://doi.org/10.1371/journal.pone.0002497
Gao, Y., Dickerson, J.B., Guo, F., Zheng, J., and Zheng, Y. (2004). Rational Design and Characterization of a Rac GTPase-Specific Small Molecule Inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618-7623. DOI: https://doi.org/10.1073/pnas.0307512101
Gateva, G., Tojkander, S., Koho, S., Carpen, O., and Lappalainen, P. (2014). Palladin Promotes Assembly of Non-Contractile Dorsal Stress Fibers through Vasp Recruitment. J. Cell Sci. 127, 1887-1898. DOI: https://doi.org/10.1242/jcs.135780
Haarer, B., and Brown, S.S. (1990). Structure and Function of Profilin.
Head, J.A., Jiang, D., Li, M., Zorn, L.J., Schaefer, E.M., Parsons, J.T., and Weed, S.A. (2003). Cortactin Tyrosine Phosphorylation Requires Rac1 Activity and Association with the Cortical Actin Cytoskeleton. Mol. Biol. Cell. 14, 3216-3229. DOI: https://doi.org/10.1091/mbc.e02-11-0753
Hotulainen, P., and Lappalainen, P. (2006). Stress Fibers are Generated by Two Distinct Actin Assembly Mechanisms in Motile Cells. J. Cell Biol. 173, 383-394. DOI: https://doi.org/10.1083/jcb.200511093
Jalal, S., Shi, S., Acharya, V., Huang, R.Y., Viasnoff, V., Bershadsky, A.D., and Tee, Y.H. (2019). Actin Cytoskeleton Self-Organization in Single Epithelial Cells and Fibroblasts under Isotropic Confinement. J. Cell Sci. 132. DOI: https://doi.org/10.1242/jcs.220780
Kwong, H.K., Huang, Y., Bao, Y., Lam, M.L., and Chen, T.H. (2019). Remnant Effects of Culture Density on Cell Chirality after Reseeding. J. Cell Sci. 132. DOI: https://doi.org/10.1242/jcs.220780
Moolenaar, W.H. (1995). Lysophosphatidic Acid, a Multifunctional Phospholipid Messenger. J. Cell Sci. 132. DOI: https://doi.org/10.1242/jcs.220780
Mukherjee, K., Ishii, K., Pillalamarri, V., Kammin, T., Atkin, J.F., Hickey, S.E., Xi, Q.J., Zepeda, C.J., Gusella, J.F., and Talkowski, M.E. (2016). Actin Capping Protein Capzb Regulates Cell Morphology, Differentiation, and Neural Crest Migration in Craniofacial Morphogenesis. Hum. Mol. Genet. 25, 1255-1270. DOI: https://doi.org/10.1093/hmg/ddw006
Sahasrabudhe, A., Ghate, K., Mutalik, S., Jacob, A., and Ghose, A. (2016). Formin 2 Regulates the Stabilization of Filopodial Tip Adhesions in Growth Cones and Affects Neuronal Outgrowth and Pathfinding In Vivo. Development 143, 449-460. DOI: https://doi.org/10.1242/dev.130104
Serrels, B., Serrels, A., Brunton, V.G., Holt, M., McLean, G.W., Gray, C.H., Jones, G.E., and Frame, M.C. (2007). Focal Adhesion Kinase Controls Actin Assembly via a Ferm-Mediated Interaction with the Arp2/3 Complex. Nat. Cell Biol. 9, 1046-1056. DOI: https://doi.org/10.1038/ncb1626
Shao, X., Li, Q., Mogilner, A., Bershadsky, A.D., and Shivashankar, G. (2015). Mechanical Stimulation Induces Formin-Dependent Assembly of a Perinuclear Actin Rim. Proc. Natl. Acad. Sci. U.S.A. 112, E2595-E2601. DOI: https://doi.org/10.1073/pnas.1504837112
Tee, Y.H., Goh, W.J., Yong, X., Ong, H.T., Hu, J., Tay, I.Y.Y., Shi, S., Jalal, S., Barnett, S.F., and Kanchanawong, P. (2023). Actin Polymerisation and Crosslinking Drive Left-Right Asymmetry in Single Cell and Cell Collectives. Nat. Commun. 14, 776. DOI: https://doi.org/10.1038/s41467-023-35918-1
Tee, Y.H., Shemesh, T., Thiagarajan, V., Hariadi, R.F., Anderson, K.L., Page, C., Volkmann, N., Hanein, D., Sivaramakrishnan, S., Kozlov, M.M., and Bershadsky, A.D. (2015). Cellular Chirality Arising from the Self-Organization of the Actin Cytoskeleton. Nat. Cell Biol. 17, 445-457. DOI: https://doi.org/10.1038/ncb3137
Tojkander, S., Gateva, G., Schevzov, G., Hotulainen, P., Naumanen, P., Martin, C., Gunning, P.W., and Lappalainen, P. (2011). A Molecular Pathway for Myosin II Recruitment to Stress Fibers. Curr. Biol. 21, 539-550. DOI: https://doi.org/10.1016/j.cub.2011.03.007
Tsuji, T., Ishizaki, T., Okamoto, M., Higashida, C., Kimura, K., Furuyashiki, T., Arakawa, Y., Birge, R.B., Nakamoto, T., Hirai, H., and Narumiya, S. (2002). Rock and mdia1 Antagonize in Rho-Dependent Rac Activation in Swiss 3T3 Fibroblasts. J. Cell Biol. 157, 819-830. DOI: https://doi.org/10.1083/jcb.200112107
Wan, L.Q., Ronaldson, K., Park, M., Taylor, G., Zhang, Y., Gimble, J.M., and Vunjak-Novakovic, G. (2011). Micropatterned Mammalian Cells Exhibit Phenotype-Specific Left-Right Asymmetry. Proc. Natl. Acad. Sci. U.S.A. 108, 12295-12300. DOI: https://doi.org/10.1073/pnas.1103834108
Witke, W. (2004). The Role of Profilin Complexes in Cell Motility and Other Cellular Processes. Trends Cell Biol. 14, 461-469. DOI: https://doi.org/10.1016/j.tcb.2004.07.003