Author Response
Public Reviews
We thank both reviewers for taking the time and effort to think critically about our paper and point out areas where it can be improved. In this document, we do our best to clarify any misunderstandings with the hope that further consideration about the strengths and weaknesses of our approach will be possible. Our responses are in bold.
Reviewer #1 (Public Review):
Summary:
In their manuscript, Schmidlin, Apodaca, et al try to answer fundamental questions about the evolution of new phenotypes and the trade-offs associated with this process. As a model, they use yeast resistance to two drugs, fluconazole and radicicol. They use barcoded libraries of isogenic yeasts to evolve thousands of strains in 12 different environments. They then measure the fitness of evolved strains in all environments and use these measurements to examine patterns in fitness trade-offs. They identify only six major clusters corresponding to different trade-off profiles, suggesting the vast genotypic landscape of evolved mutants translates to a highly constrained phenotypic space. They sequence over a hundred evolved strains and find that mutations in the same gene can result in different phenotypic profiles.
Overall, the authors deploy innovative methods to scale up experimental evolution experiments, and in many aspects of their approach tried to minimize experimental variation.
We thank the reviewer for this positive assessment of our work. We are happy that the reviewer noted what we feel is a unique strength of our approach: we scaled up experimental evolution by using DNA barcodes and by exploring 12 related selection pressures. Despite this scaling up, we still see phenotypic convergence among the 744 adaptive mutants we study.
The environments we study represent 12 different concentrations or combinations of two drugs, radicicol and fluconazole. Our hope is that this large dataset (774 mutants x 12 environments) will be useful, both to scientists who are generally interested in the genetic and phenotypic underpinnings of adaptation, and to scientists specifically interested in the evolution of drug resistance.
Weaknesses:
(1) One of the objectives of the authors is to characterize the extent of phenotypic diversity in terms of resistance trade-offs between fluconazole and radicicol. To minimize noise in the measurement of relative fitness, the authors only included strains with at least 500 barcode counts across all time points in all 12 experimental conditions, resulting in a set of 774 lineages passing this threshold. This corresponds to a very small fraction of the starting set of ~21 000 lineages that were combined after experimental evolution for fitness measurements.
This is a misunderstanding that we will work to clarify in the revision. Our starting set did not include 21,000 adaptive lineages. The total number of unique adaptive lineages in this starting set is much lower than 21,000 for two reasons.
First, ~21,000 represents the number of single colonies we isolated in total from our evolution experiments. Many of these isolates possess the same barcode, meaning they are duplicates. Second, and more importantly, most evolved lineages do not acquire adaptive mutations, meaning that many of the 21,000 isolates are genetically identical to their ancestor. In our revised manuscript, we will explicitly state that these 21,000 isolated lineages do not all represent unique, adaptive lineages. In figure 2 and all associated text, we will change the word “lineages” to “isolates,” where relevant.
More broadly speaking, several previous studies have demonstrated that diverse genetic mutations converge at the level of phenotype, and have suggested that this convergence makes adaptation more predictable (PMID33263280, PMID37437111, PMID22282810, PMID25806684). Our study captures mutants that are overlooked in previous studies, such as those that emerge across subtly different selection pressures (e.g., 4 𝜇g/ml vs. 8 𝜇g/ml flu) and those that are undetectable in evolutions lacking DNA barcodes. Thus, while our experimental design misses some mutants (see next comment), it captures many others. Note that 774 adaptive lineages is more than most previous studies. Thus, we feel that “our work – showing that 774 mutants fall into a much smaller number of groups” is important because it “contributes to growing literature suggesting that the phenotypic basis of adaptation is not as diverse as the genetic basis (lines 161 - 162).”
As the authors briefly remark, this will bias their datasets for lineages with high fitness in all 12 environments, as all these strains must be fit enough to maintain a high abundance.
The word “briefly” feels a bit unfair because we discuss this bias on 3 separate occasions (on lines 146 - 147, 260 - 264, and in more detail on 706 - 714). We even walk through an example of a class of mutants that our study misses. We say, “our study is underpowered to detect adaptive lineages that have low fitness in any of the 12
environments. This is bound to exclude large numbers of adaptive mutants. For example, previous work has shown some FLU resistant mutants have strong tradeoffs in RAD (Cowen and Lindquist 2005). Perhaps we are unable to detect these mutants because their barcodes are at too low a frequency in RAD environments, thus they are excluded from our collection of 774.”
In our revised version, we will add more text to the first mention of these missing mutants (lines 146 - 147) so that the implications are more immediately made apparent.
While we “miss” some classes of mutants, we “catch” other classes that may have been missed in previous studies of convergence. For example, we observe a unique class of
FLU-resistant mutants that primarily emerged in evolution experiments that lack FLU
(Figure 3). Thus, we think that the unique design of our study, surveying 12 environments, allows us to make a novel contribution to the study of phenotypic convergence.
One of the main observations of the authors is phenotypic space is constrained to a few clusters of roughly similar relative fitness patterns, giving hope that such clusters could be enumerated and considered to design antimicrobial treatment strategies. However, by excluding all lineages that fit in only one or a few environments, they conceal much of the diversity that might exist in terms of trade-offs and set up an inclusion threshold that might present only a small fraction of phenotypic space with characteristics consistent with generalist resistance mechanisms or broadly increased fitness. This has important implications regarding the general conclusions of the authors regarding the evolution of trade-offs.
We discussed these implications in some detail in the 16 lines mentioned above (146 - 147, 260 - 264, 706 - 714). To add to this discussion, we will also add the following sentence to the end of the paragraph on lines 697 - 714: “This could complicate (or even make impossible) endeavors to design antimicrobial treatment strategies that thwart resistance”.
We will also add a new paragraph that discusses these implications earlier in our manuscript. This paragraph will highlight the strengths of our method (e.g., that we “catch” classes of mutants that are often overlooked) while being transparent about the weaknesses of our approach (e.g., that we “miss” mutants with strong tradeoffs).
(2) Most large-scale pooled competition assays using barcodes are usually stopped after ~25 to avoid noise due to the emergence of secondary mutations.
The rate at which new mutations enter a population is driven by various factors such as the mutation rate and population size, so choosing an arbitrary threshold like 25 generations is difficult.
We conducted our fitness competition following previous work using the Levy/Blundell yeast barcode system, in which the number of generations reported varies from 32 to 40 (PMID33263280, PMID27594428, PMID37861305, see PMID27594428 for detailed calculation of the fraction of lineages biased by secondary mutations in this system).
The authors measure fitness across ~40 generations, which is almost the same number of generations as in the evolution experiment. This raises the possibility of secondary mutations biasing abundance values, which would not have been detected by the whole genome sequencing as it was performed before the competition assay.
We understand how the reviewer came to this misunderstanding and will adjust our revised manuscript accordingly. Previous work has demonstrated that, in this particular evolution platform, most of the mutations actually occur during the transformation that introduces the DNA barcodes (PMID25731169). In other words, these mutations do not accumulate during the 40 generations of evolution, they are already there. So the observation that we collect a genetically diverse pool of adaptive mutants after 40 generations of evolution is not evidence that 40 generations is enough time for secondary mutations to bias abundance values.
(3) The approach used by the authors to identify and visualize clusters of phenotypes among lineages does not seem to consider the uncertainty in the measurement of their relative fitness. As can be seen from Figure S4, the inter-replicate difference in measured fitness can often be quite large. From these graphs, it is also possible to see that some of the fitness measurements do not correlate linearly (ex.: Med Flu, Hi Rad Low Flu), meaning that taking the average of both replicates might not be the best approach.
This concern, and all subsequent concerns, seem to be driven by either (a) general concerns about the noisiness of fitness measurements obtained from large-scale barcode fitness assays or (b) general concerns about whether the clusters obtained from our dimensional reduction approach capture this noise as opposed to biologically meaningful differences.
We will respond to each concern point-by-point, but want to start by generally stating that (a) our particular large-scale barcode fitness assay has several features that diminish noise, and (b) we devote 4 figures and 200 lines of text to demonstrating that these clusters capture biologically meaningful differences between mutants (and not noise).
In terms of this specific concern, we performed an analysis of noise in the submitted manuscript: Our noisiest fitness measurements correspond to barcodes that are the least abundant and thus suffer the most from stochastic sampling noise. These are also the barcodes that introduce the nonlinearity the reviewer mentions. We removed these from our dataset by increasing our coverage threshold from 500 reads to 5,000 reads. The clusters did not collapse, which suggests that they were not capturing noise (Figure S7 panel B). But we agree with the reviewer that this analysis alone is not sufficient to conclude that the clusters distinguish groups of mutants with unique fitness tradeoffs.
Because the clustering approach used does not seem to take this variability into account, it becomes difficult to evaluate the strength of the clustering, especially because the UMAP projection does not include any representation of uncertainty around the position of lineages.
To evaluate the strength of the clustering, we performed numerous analyses including whole genome sequencing, growth experiments, reclustering, and tracing the evolutionary origins of each cluster (Figures 5 - 8). All of these analyses suggested that our clusters capture groups of mutants that have different fitness tradeoffs. We will adjust our revised manuscript to make clear that we do not rely on the results of a clustering algorithm alone to draw conclusions about phenotypic convergence.
We are also grateful to the reviewer for helping us realize that, as written, our manuscript is not clear with regard to how we perform clustering. We are not using UMAP to decide which mutant belongs to which cluster. Recent work highlights the importance of using an independent clustering method (PMID37590228). Although this recent work addresses the challenge of clustering much higher dimensional data than we survey here, we did indeed use an independent clustering method (gaussian mixture model). In other words, we use UMAP for visualization but not clustering. We also confirm our clustering results using a second independent method (hierarchical clustering; Figure S8). And in our revised manuscript, will confirm with a third method (PCA, see below). We will adjust the main text and the methods section to make these choices clearer.
This might paint a misleading picture where clusters appear well separate and well defined but are in fact much fuzzier, which would impact the conclusion that the phenotypic space is constricted.
The salient question is whether the clusters are so “fuzzy” that they are not meaningful. That interpretation seems unreasonable. Our clusters group mutants with similar genotypes, evolutionary histories, and fitness tradeoffs (Figures 5 - 8). Clustering mutants with similar behaviors is important and useful. It improves phenotypic prediction by revealing which mutants are likely to have at least some phenotypic effects in common. And it also suggests that the phenotypic space is constrained, at least to some degree, which previous work suggests is helpful in predicting evolution (PMID33263280, PMID37437111, PMID22282810, PMID25806684).
(4) The authors make the decision to use UMAP and a gaussian mixed model to cluster and represent the different fitness landscapes of their lineages of interest. Their approach has many caveats. First, compared to PCA, the axis does not provide any information about the actual dissimilarities between clusters. Using PCA would have allowed a better understanding of the amount of variance explained by components that separate clusters, as well as more interpretable components.
The components derived from PCA are often not interpretable. It’s not obvious that each one, or even the first one, will represent some intuitive phenotype, like resistance to fluconazole.
Moreover, we see many non-linearities in our data. For example, fitness in a double drug environment is not predicted by adding up fitness in the relevant single drug environments. Also, there are mutants that have high fitness when fluconazole is absent or abundant, but low fitness when mild concentrations are present. These types of nonlinearities can make the axes in PCA very difficult to interpret, plus these nonlinearities can be missed by PCA, thus we prefer other clustering methods.
We will adjust our revised manuscript to explain these reasons why we chose UMAP and GMM over PCA.
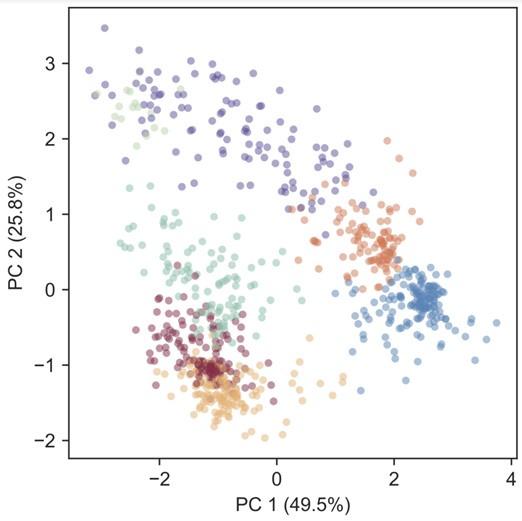
Also, we will include PCA in the supplement of our revised manuscript. Please find below PC1 vs PC2, with points colored according to the cluster assignment in figure 4 (i.e.
using a gaussian mixture model). It appears the clusters are largely preserved.
Author response image 1.
Second, the advantages of dimensional reduction are not clear. In the competition experiment, 11/12 conditions (all but the no drug, no DMSO conditions) can be mapped to only three dimensions: concentration of fluconazole, concentration of radicicol, and relative fitness. Each lineage would have its own fitness landscape as defined by the plane formed by relative fitness values in this space, which can then be examined and compared between lineages.
We worry that the idea stems from apriori notions of what the important dimensions should be. It also seems like this would miss important nonlinearities such as our observation that low fluconazole behaves more like a novel selection pressure than a dialed down version of high fluconazole.
Also, we believe the reviewer meant “fitness profile” and not “fitness landscape”. A fitness landscape imagines a walk where every “step” is a mutation. Most lineages in barcoded evolution experiments possess only a single adaptive mutation. A single-step walk is not enough to build a landscape, though others are expanding barcoded evolution experiments beyond the first step (PMID34465770, PMID31723263), so maybe one day this will be possible.
Third, the choice of 7 clusters as the cutoff for the multiple Gaussian model is not well explained. Based on Figure S6A, BIC starts leveling off at 6 clusters, not 7, and going to 8 clusters would provide the same reduction as going from 6 to 7. This choice also appears arbitrary in Figure S6B, where BIC levels off at 9 clusters when only highly abundant lineages are considered.
We agree. We did not rely on the results of BIC alone to make final decisions about how many clusters to include. We thank the reviewer for pointing out this gap in our writing. We will adjust our revised manuscript to explain that we ultimately chose to describe 6 clusters that we were able to validate with follow-up experiments. In figures 5, 6, 7, and 8, we use external information to validate the clusters that we report in figure 4. And in lines 697 – 714, we explain that there are may be additional clusters beyond those we tease apart in this study.
This directly contradicts the statement in the main text that clusters are robust to noise, as more a stringent inclusion threshold appears to increase and not decrease the optimal number of clusters. Additional criteria to BIC could have been used to help choose the optimal number of clusters or even if mixed Gaussian modeling is appropriate for this dataset.
We are under the following impression: If our clustering method was overfitting, i.e. capturing noise, the optimal number of clusters should decrease when we eliminate noise. It increased. In other words, the observation that our clusters did not collapse (i.e.
merge) when we removed noise suggests these clusters were not capturing noise.
More generally, our validation experiments, described below, provide additional evidence that our clusters capture meaningful differences between mutants (and not noise).
(5) Large-scale barcode sequencing assays can often be noisy and are generally validated using growth curves or competition assays.
Some types of bar-seq methods, in particular those that look at fold change across two time points, are noisier than others that look at how frequency changes across multiple timepoints (PMID30391162). Here, we use the less noisy method. We also reduce noise by using a stricter coverage threshold than previous work (e.g., PMID33263280), and by excluding batch effects by performing all experiments simultaneously (PMID37237236).
The main assay we use to measure fitness has been previously validated
(PMID27594428). No subsequent study using this assay validates using the methods suggested by the reviewer (see PMID37861305, PMID33263280, PMID31611676, PMID29429618, PMID37192196, PMID34465770, PMID33493203).
More to the point, bar-seq has been used, without the reviewer’s suggested validation, to demonstrate that the way some mutant’s fitness changes across environments is different from other mutants (PMID33263280, PMID37861305, PMID31611676, PMID33493203, PMID34596043). This is the same thing that we use bar-seq to demonstrate.
For all of these reasons, we are hesitant to confirm bar-seq itself as a valid way to infer fitness. It seems this is already accepted as a standard in our field.
Having these types of results would help support the accuracy of the main assay in the manuscript and thus better support the claims of the authors.
We don’t agree that fitness measurements obtained from this bar-seq assay generally require validation. But we do agree that it is important to validate whether the mutants in each of our 6 clusters indeed are different from one another in meaningful ways, in particular, in that they have different fitness tradeoffs. We have four figures (5 - 8) and 200 lines of text dedicated to validating whether our clusters capture reproducible and biologically meaningful differences between mutants. Happily, one of these figures (Fig 7) includes growth curves, which are exactly the type of validation experiment asked for by the reviewer.
Below, we walk through the different types of validation experiments that are present in our original manuscript, and additional validation experiments that we plan to include in the revised version. We are hopeful that these validation experiments are sufficient, or at the very least, that this list empowers reviewers to point out where more work is needed.
(1) Mutants from different clusters have different growth curves: In our original manuscript, we measured growth curves corresponding to a fitness tradeoff that we thought was surprising. Mutants in clusters 4 and 5 both have fitness advantages in single drug conditions. While mutants from cluster 4 also are advantageous in the double drug conditions, mutants from cluster 5 are not! We validated these different behaviors by studying growth curves for a mutant from each cluster (Figures 7 and S10).
(2) Mutants from different clusters have different evolutionary origins: In our original manuscript, we came up with a novel way to ask whether the clusters capture different types of adaptive mutants. We asked whether the mutants in each cluster originate from different evolution experiments. Indeed they often do (see pie charts in Figures 6, 7, 8). This method also provides evidence supporting each cluster’s differing fitness tradeoffs.
For example, mutants in cluster 5 appear to have a tradeoff in a double drug condition (described above). They rarely originate from that evolution condition, unlike mutants in nearby cluster 4 (see Figure 7).
(3) Mutants from each cluster often fall into different genes: In our original manuscript, we sequenced many of these mutants and show that mutants in the same gene are often found in the same cluster. For example, all 3 IRA1 mutants are in cluster 6 (Fig 8), both GPB2 mutants are in cluster 4 (Figs 7 & 8), and 35/36 PDR mutants are in either cluster 2 or 3 (Figs 5 & 6).
(4) Mutants from each cluster have behaviors previously observed in the literature: In our original manuscript, we compared our sequencing results to the literature and found congruence. For example, PDR mutants are known to provide a fitness benefit in fluconazole and are found in clusters that have high fitness in fluconazole (lines 457 - 462). Previous work suggests that some mutations to PDR have different tradeoffs than others, which is what we see (lines 540 - 542). IRA1 mutants were previously observed to have high fitness in our “no drug” condition, and are found in the cluster that has the highest fitness in the “no drug” condition (lines 642 - 646). Previous work even confirms the unusual fitness tradeoff we observe where IRA1 and other cluster 6 mutants have low fitness only in low concentrations of fluconazole (lines 652 - 657).
(5) Mutants largely remain in their clusters when we use alternate clustering methods: In our original manuscript, we performed various different reclustering and/or normalization approaches on our data (Fig 6, S5, S7, S8, S9). The clusters of mutants that we observe in figure 4 do not change substantially when we recluster the data. We will add PCA (see above) to these analyses in our revised manuscript.
(6) We will include additional data showing that mutants in different clusters have different evolutionary origins: Cluster 1 is defined by high fitness in low fluconazole that declines with increasing fluconazole (see Fig 4E and Fig 5C). In our revised manuscript, we will show that cluster 1 lineages were overwhelmingly sampled from evolutions conducted in our lowest concentration of fluconazole (see figure panel A below). No other cluster’s evolutionary history shows this pattern (figures 6, 7, and 8).
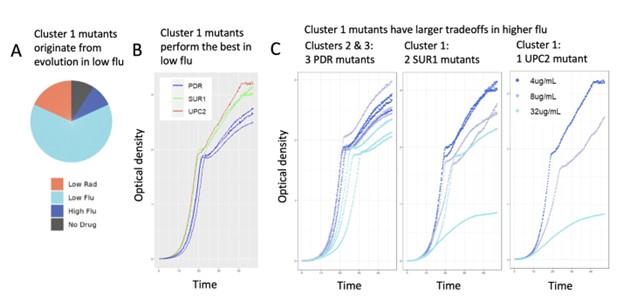
(7) We will include additional data showing that mutants in different clusters have different growth curves: Cluster 1 lineages are unique in that their fitness advantage is specific to low flu and trades off in higher concentrations of fluconazole. We obtained growth curves for three cluster 1 mutants (2 SUR1 mutants and 1 UPC2 mutant). We compared them to growth curves for three PDR mutants (from clusters 2 and 3). Cluster 1 mutants appear to have the highest growth rates and reach the higher carrying capacity in low fluconazole (see red and green lines in Author response image 2 panel B below). But the cluster 1 mutants are negatively affected by higher concentrations of fluconazole, much more so than the mutants from clusters 2 and 3 (see Author response image 2 panel C below). This is consistent with the different fitness tradeoffs we observe for each cluster (figures 4 and 5). We will include a more detailed version of this analysis and the figures below in our revised manuscript.
Author response image 2.
Validation experiments demonstrate that cluster 1 mutants have uniquely high fitness in only the lowest concentration of fluconazole. (A) The mutant lineages in cluster 1 were largely sampled from evolution experiments performed in low flu. This is not true of other clusters (see pie charts in main manuscript). (B) In low flu (4 𝜇g/ml), Cluster 1 lineages (red/UPC2 and green/SUR1) grow faster and achieve higher density than lineages from clusters 2 and 3 (blue/PDR). This is consistent with barseq measurements demonstrating that cluster 1 mutants have the highest fitness in low flu. (C) Cluster 1 lineages are sensitive to increasing flu concentrations (SUR1 and UPC2 mutants, middle and rightmost graphs). This is apparent in that the gray (8 𝜇g/ml flu) and light blue (32 𝜇g/ml flu) growth curves rise more slowly and reach lower density than the dark blue curves (4 𝜇g/ml flu). But this is not the case for the PDR mutants from clusters 2 and 3 (leftmost graph). These observations are consistent with the bar-seq fitness data presented in the main manuscript (Fig 4E).
With all of these validation efforts combined, we are hopeful that the reviewer is now more convinced that our clusters capture groups of mutants with different fitness tradeoffs (as opposed to noise). We want to conclude by saying that we are grateful to the reviewer for making us think deeply about areas where we can include additional validation efforts as well as areas where we can make our manuscript clearer.
Reviewer #2 (Public Review):
Summary:
Schmidlin & Apodaca et al. aim to distinguish mutants that resist drugs via different mechanisms by examining fitness tradeoffs across hundreds of fluconazole-resistant yeast strains. They barcoded a collection of fluconazole-resistant isolates and evolved them in different environments with a view to having relevance for evolutionary theory, medicine, and genotypephenotype mapping.
Strengths:
There are multiple strengths to this paper, the first of which is pointing out how much work has gone into it; the quality of the experiments (the thought process, the data, the figures) is excellent. Here, the authors seek to induce mutations in multiple environments, which is a really large-scale task. I particularly like the attention paid to isolates with are resistant to low concentrations of FLU. So often these are overlooked in favour of those conferring MIC values >64/128 etc. What was seen is different genotype and fitness profiles. I think there's a wealth of information here that will actually be of interest to more than just the fields mentioned (evolutionary medicine/theory).
We are very grateful for this positive review. This was indeed a lot of work! We are happy that the reviewer noted what we feel is a unique strength of our manuscript: that we survey adaptive isolates across multiple environments, including low drug concentrations.
Weaknesses:
Not picking up low fitness lineages - which the authors discuss and provide a rationale as to why. I can completely see how this has occurred during this research, and whilst it is a shame I do not think this takes away from the findings of this paper. Maybe in the next one!
We thank the reviewer for these words of encouragement and will work towards catching more low fitness lineages in our next project.
In the abstract the authors focus on 'tradeoffs' yet in the discussion they say the purpose of the study is to see how many different mechanisms of FLU resistance may exist (lines 679-680), followed up by "We distinguish mutants that likely act via different mechanisms by identifying those with different fitness tradeoffs across 12 environments". Whilst I do see their point, and this is entirely feasible, I would like a bit more explanation around this (perhaps in the intro) to help lay-readers make this jump. The remainder of my comments on 'weaknesses' are relatively fixable, I think:
We think that phrasing the “jump” as a question might help lay readers get from point A to point B. So, in the introduction of our revised manuscript, we will add a paragraph roughly similar to this one: “If two groups of drug-resistant mutants have different fitness tradeoffs, does it mean that they provide resistance through different underlying mechanisms? Alternatively, it could mean that both provide drug resistance via the same mechanism, but some mutations come with a cost that others don’t pay. However, another way to phrase this alternative is to say that both groups of mutants affect fitness through different suites of mechanisms that are only partially overlapping. And so, by identifying groups of mutants with different fitness tradeoffs, we argue that we will be uncovering sets of mutations that impact fitness through different underlying mechanisms. The ability to do so would be useful for genotype-phenotype mapping endeavors.”
In the introduction I struggle to see how this body of research fits in with the current literature, as the literature cited is a hodge-podge of bacterial and fungal evolution studies, which are very different! So example, the authors state "previous work suggests that mutants with different fitness tradeoffs may affect fitness through different molecular mechanisms" (lines 129-131) and then cite three papers, only one of which is a fungal research output. However, the next sentence focuses solely on literature from fungal research. Citing bacterial work as a foundation is fine, but as you're using yeast for this I think tailoring the introduction more to what is and isn't known in fungi would be more appropriate. It would also be great to then circle back around and mention monotherapy vs combination drug therapy for fungal infections as a rationale for this study. The study seems to be focused on FLU-resistant mutants, which is the first-line drug of choice, but many (yeast) infections have acquired resistance to this and combination therapy is the norm.
In our revised manuscript, we will carefully review all citations. The issue may stem from our attempt to reach two different groups of scientists. We ourselves are broadly interested in the structure of the genotype-phenotype-fitness map (PMID33263280, PMID32804946). Though the 3 papers the reviewer mentions on lines 132 - 133 all pertain to yeast, we cite them because they are studies about the complexity of this map. Their conclusions, in theory, should apply broadly, beyond yeast. Similarly, the reason we cite papers from yeast, as well as bacteria and cancer, is that we believe general conclusions about the genotype-phenotype-fitness map should apply broadly. For example, the sentence the reviewer highlights, “previous work suggests that mutants with different fitness tradeoffs may affect fitness through different molecular mechanisms” is a general observation about the way genotype maps to fitness. So we cited papers from across the tree of life to support this sentence.
On the other hand, because we study drug resistant mutations, we also hope that our work is of use to scientists studying the evolution of resistance. We agree with the reviewer that in this regard, some of our findings may be especially pertinent to the evolution of resistance to antifungal drugs. We will consider this when reviewing the citations in our revised manuscript and add some text to clarify these points.
Methods: Line 769 - which yeast? I haven't even seen mention of which species is being used in this study; different yeast employ different mechanisms of adaptation for resistance, so could greatly impact the results seen. This could help with some background context if the species is mentioned (although I assume S. cerevisiae).
In the revised manuscript, we will make clear that we study S. cerevisiae.
In which case, should aneuploidy be considered as a mechanism? This is mentioned briefly on line 556, but with all the sequencing data acquired this could be checked quickly?
We like this idea and we are working on it, but it is not straightforward. The reviewer is correct in that we can use the sequencing data that we already have. But calling aneuploidy with certainty is tough because its signal can be masked by noise. In other words, some regions of the genome may be sequenced more than others by chance.
Given this is not straightforward, at least not for us, this analysis will likely have to wait for a subsequent paper.
I think the authors could be bolder and try and link this to other (pathogenic) yeasts. What are the implications of this work on say, Candida infections?
Perhaps because our background lies in general study of the genotype-phenotype map, we did not want to make bold assertions about how our work might apply to pathogenic yeasts. But we see how this could be helpful and will add some discussion points about this. Specifically, we will discuss which of the genes and mutants we observe are also found in Candida. We will also investigate whether our observation that low fluconazole represents a seemingly unique challenge, not just a milder version of high fluconazole, has any corollary in the Candida literature.





