Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorAdrien PeyracheMcGill University, Montreal, Canada
- Senior EditorMichael FrankBrown University, Providence, United States of America
Reviewer #1 (Public Review):
Summary:
This paper investigates the neural population activity patterns of the medial frontal cortex in rats performing a nose poking timing task using in vivo calcium imaging. The results showed neurons that were active at the beginning and end of the nose poking and neurons that formed sequential patterns of activation that covaried with the timed interval during nose poking on a trial-by-trial basis. The former were not stable across sessions, while the latter tended to remain stable over weeks. The analysis on incorrect trials suggests the shorter non-rewarded intervals were due to errors in the scaling of the sequential pattern of activity.
Strengths:
This study measured stable signals using in vivo calcium imaging during experimental sessions that were separated by many days in animals performing a nose poking timing task. The correlation analysis on the activation profile to separate the cells in the three groups was effective and the functional dissociation between beginning and end, and duration cells was revealing. The analysis on the stability of decoding of both the nose poking state and poking time was very informative. Hence, this study dissected a neural population that formed sequential patterns of activation that encoded timed intervals.
Weaknesses:
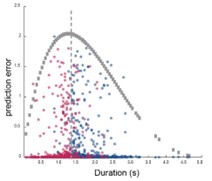
It is not clear whether animals had enough simultaneously recorded cells to perform the analyzes of Figures 2-4. In fact, rat 3 had 18 responsive neurons which probably is not enough to get robust neural sequences for the trial-by-trial analysis and the correct and incorrect trial analysis. In addition, the analysis of behavioral errors could be improved. The analysis in Figure 4A could be replaced by a detailed analysis on the speed, and the geometry of neural population trajectories for correct and incorrect trials. In the case of Figure 4G is not clear why the density of errors formed two clusters instead of having a linear relation with the produce duration. I would be recommendable to compute the scaling factor on neuronal population trajectories and single cell activity or the computation of the center of mass to test the type III errors.
Due to the slow time resolution of calcium imaging, it is difficult to perform robust analysis on ramping activity. Therefore, I recommend downplaying the conclusion that: "Together, our data suggest that sequential activity might be a more relevant coding regime than the ramping activity in representing time under physiological conditions."
Reviewer #2 (Public Review):
In this manuscript, Li and collaborators set out to investigate the neuronal mechanisms underlying "subjective time estimation" in rats. For this purpose, they conducted calcium imaging in the prefrontal cortex of water-restricted rats that were required to perform an action (nosepoking) for a short duration to obtain drops of water. The authors provided evidence that animals progressively improved in performing their task. They subsequently analyzed the calcium imaging activity of neurons and identify start, duration, and stop cells associated with the nose poke. Specifically, they focused on duration cells and demonstrated that these cells served as a good proxy for timing on a trial-by-trial basis, scaling their pattern of actvity in accordance with changes in behavioral performance. In summary, as stated in the title, the authors claim to provide mechanistic insights into subjective time estimation in rats, a function they deem important for various cognitive conditions.
This study aligns with a wide range of studies in system neuroscience that presume that rodents solve timing tasks through an explicit internal estimation of duration, underpinned by neuronal representations of time. Within this framework, the authors performed complex and challenging experiments, along with advanced data analysis, which undoubtedly merits acknowledgement. However, the question of time perception is a challenging one, and caution should be exercised when applying abstract ideas derived from human cognition to animals. Studying so-called time perception in rats has significant shortcomings because, whether acknowledged or not, rats do not passively estimate time in their heads. They are constantly in motion. Moreover, rats do not perform the task for the sake of estimating time but to obtain their rewards are they water restricted. Their behavior will therefore reflects their motivation and urgency to obtain rewards. Unfortunately, it appears that the authors are not aware of these shortcomings. These alternative processes (motivation, sensorimotor dynamics) that occur during task performance are likely to influence neuronal activity. Consequently, my review will be rather critical. It is not however intended to be dismissive. I acknowledge that the authors may have been influenced by numerous published studies that already draw similar conclusions. Unfortunately, all the data presented in this study can be explained without invoking the concept of time estimation. Therefore, I hope the authors will find my comments constructive and understand that as scientists, we cannot ignore alternative interpretations, even if they conflict with our a priori philosophical stance (e.g., duration can be explicitly estimated by reading neuronal representation of time) and anthropomorphic assumptions (e.g., rats estimate time as humans do). While space is limited in a review, if the authors are interested, they can refer to a lengthy review I recently published on this topic, which demonstrates that my criticism is supported by a wide range of timing experiments across species (Robbe, 2023). In addition to this major conceptual issue that cast doubt on most of the conclusions of the study, there are also several major statistical issues.
Main Concerns
(#1) The authors used a task in which rats must poke for a minimal amount of time (300 ms and then 1500 ms) to be able to obtain a drop of water delivered a few centimeters right below the nosepoke. They claim that their task is a time estimation task. However, they forget that they work with thirsty rats that are eager to get water sooner than later (there is a reason why they start by a short duration!). This task is mainly probing the animals ability to wait (that is impulse control) rather than time estimation per se. Second, the task does not require to estimate precisely time because there appear to be no penalties when the nosepokes are too short or when they exceed. So it will be unclear if the variation in nosepoke reflects motivational changes rather than time estimation changes. The fact that this behavioral task is a poor assay for time estimation and rather reflects impulse control is shown by the tendency of animals to perform nose-pokes that are too short, the very slow improvement in their performance (Figure 1, with most of the mice making short responses), and the huge variability. Not only do the behavioral data not support the claim of the authors in terms of what the animals are actually doing (estimating time), but this also completely annhilates the interpretation of the Ca++ imaging data, which can be explained by motivational factors (changes in neuronal activity occurring while the animals nose poke may reflect a growing sens of urgency to check if water is available).
(#2) A second issue is that the authors seem to assume that rats are perfectly immobile and perform like some kind of robots that would initiate nose pokes, maintain them, and remove them in a very discretized manner. However, in this kind of task, rats are constantly moving from the reward magazine to the nose poke. They also move while nose-poking (either their body or their mouth), and when they come out of the nose poke, they immediately move toward the reward spout. Thus, there is a continuous stream of movements, including fidgeting, that will covary with timing. Numerous studies have shown that sensorimotor dynamics influence neural activity, even in the prefrontal cortex. Therefore, the authors cannot rule out that what the records reflect are movements (and the scaling of movement) rather than underlying processes of time estimation (some kind of timer). Concretely, start cells could represent the ending of the movement going from the water spout to the nosepoke, and end cells could be neurons that initiate (if one can really isolate any initiation, which I doubt) the movement from the nosepoke to the water spout. Duration cells could reflect fidgeting or orofacial movements combined with an increasing urgency to leave the nose pokes.
(#3) The statistics should be rethought for both the behavioral and neuronal data. They should be conducted separately for all the rats, as there is likely interindividual variability in the impulsivity of the animals.
(#4) The fact that neuronal activity reflects an integration of movement and motivational factors rather than some abstract timing appears to be well compatible with the analysis conducted on the error trials (Figure 4), considering that the sensorimotor and motivational dynamics will rescale with the durations of the nose poke.
(#5) The authors should mention upfront in the main text (result section) the temporal resolution allowed by their Ca+ probe and discuss whether it is fast enough in regard of behavioral dynamics occurring in the task.