Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
Weaknesses:
The weaknesses of the study include the following.
(1) It remains unclear whether the function described for CDK2 is regulatory, that is, it affects TBK1 levels during physiological responses such as viral infection or cell cycle progression, or if it is homeostatic, governing the basal abundance of TBK1 but not responding to signaling.
The regulation of TBK1 by CDK2 described in this article occurs during viral infection. Simultaneously, we also investigated the effects of CDK2 overexpression and knockdown on TBK1 levels under non-infected state and observed a slight reduction, as shown in Figure 4K and 4L. Thus, we speculate that the regulation of TBK1 by CDK2 serves, on one hand, to maintain cellular homeostasis and, on the other hand, to respond to signaling triggered by viral infection.
(2) The authors have not explored whether the catalytic activity of CDK2 is required for TBK1 ubiquitinoylation and, if so, what its target specificity is.
We found that the ubiquitination modification of TBK1 was not affected by treatment with a CDK2 kinase activity inhibitor (SNS-032), as demonstrated in the results below (Author response image 1).
Author response image 1.
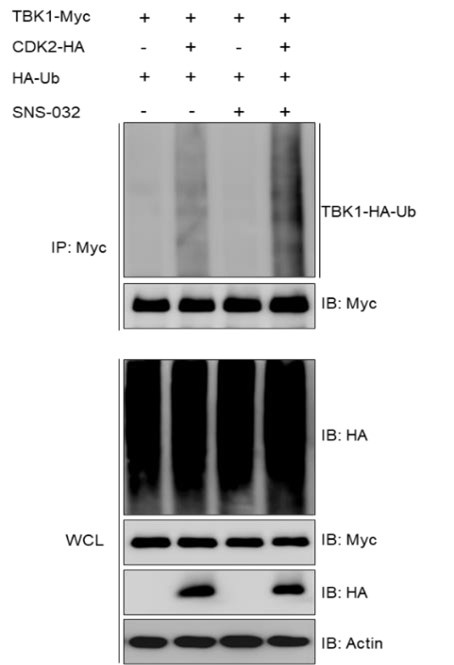
(3) Given the multitude of CDK isoforms in fish, it remains unexplored whether the identified fish CDK2 homolog is a requisite cell cycle regulator or if its action in the cell cycle is redundant with other CDKs.
A comparison of the protein sequences of fish CDK2 and human CDK2 revealed a 90% similarity (Author response image 2). It has also been reported that the kinase activity of goldfish CDK2 significantly increases during oocyte maturation (ref. 1). Furthermore, UHRF1 phosphorylation by cyclin A2/CDK2 is crucial for zebrafish embryogenesis (ref. 2). Additionally, Red grouper nervous necrosis virus (RGNNV) infection activated the p53 pathway, leading to the upregulation of p21 and downregulation of cyclin E and CDK2, which forces infected cells to remain in the G1/S replicative phase (ref. 3). All these evidences suggest that fish CDK2 plays a vital role in cell cycle regulation, and there have been no reports of other CDKs demonstrating CDK2-like functions.
References:
(1) Hirai T, et al. (1992) Isolation and Characterization of Goldfish Cdk2, a Cognate Variant of the Cell-Cycle Regulator Cdc2. Developmental biology 152(1):113-120.
(2) Chu J, et al. (2012) UHRF1 phosphorylation by cyclin A2/cyclin-dependent kinase 2 is required for zebrafish embryogenesis. Molecular biology of the cell 23(1):59-70.
(3) Mai WJ, Liu HX, Chen HQ, Zhou YJ, & Chen Y (2018) RGNNV-induced cell cycle arrest at G1/S phase enhanced viral replication via p53-dependent pathway in GS cells. Virus Res 256:142-152.
Author response image 2.
Reviewer #2 (Public Review):
Weaknesses:
(1) While the study focuses on fish, the broader implications for other lower vertebrates and higher vertebrates are not extensively discussed.
Thanks to your comment, we have added a paragraph to the Discussion section of the manuscript regarding the implications of the negative regulation of IFN expression by fish CDK2 for other vertebrates (lines 398-403). The details are as follows: first, we selected representative species from each of the six major vertebrate groups and compared their CDK2 protein sequences, finding that they are over 90% similar to one another (Author response image 3). This suggests that the function of CDK2 may be conserved to some extent across vertebrates. Additionally, CDK2 inhibition has been shown to enhance anti-tumor immunity by increasing the IFN response to endogenous retroviruses (ref. 1). Our studies provide evidence that fish CDK2 inhibits the IFN response by promoting the ubiquitination and degradation of TBK1, strongly supporting the role of CDK2 in the regulation of the immune response.
Reference:
(1) Chen Y, et al. (2022) CDK2 Inhibition Enhances Antitumor Immunity by Increasing IFN Response to Endogenous Retroviruses. Cancer Immunol Res 10(4):525-539.
Author response image 3.
(2) The study heavily relies on specific fish models, which may limit the generalizability of the findings across different species.
Thank you for your comment. First, we compared the amino acid sequences of CDK2 proteins from fish and other vertebrates, which show over 90% similarity. Moreover, the small size, low cost, and external development of zebrafish make it an excellent model for vertebrate developmental biology. It has been reported that due to the high genomic and molecular similarities between zebrafish and other vertebrates, including humans, many significant discoveries in zebrafish development are relevant to humans (ref. 2). Our study concentrated on CDK2 in zebrafish, and the findings should be valuable for other vertebrates.
Reference:
(2) Veldman MB & Lin S (2008) Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr Res 64(5):470-476.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
The following additional data/discussion could improve the manuscript.
(1) Investigate whether the catalytic activity of CDK2 is required to regulate TBK1 abundance. It is common for E3 ligases to be directed towards phosphorylated substrates, so it would be of interest to know if CDK2 phosphorylates TBK1 to facilitate its recognition for ubiquitinylation.
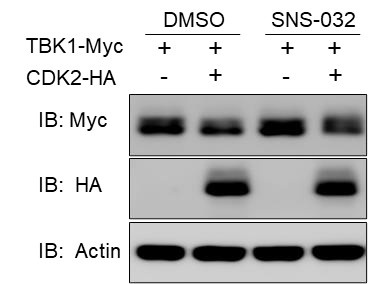
We examined the effect of CDK2 on the TBK1 protein after inhibiting its kinase activity with SNS-032 treatment and found that it could still affect TBK1 expression, as shown in the results below (Figure R4). Our previous experiments investigating the effect of CDK2 on TBK1 did not show that CDK2 caused the migration of TBK1 bands (typically, proteins that undergo phosphorylation exhibit band migration). Furthermore, in this study, CDK2 did not function as an E3 ligase; instead, it recruited the E3 ligase Dtx4 to ubiquitinate TBK1.
Author response image 4.
(2) Investigate how CDK2 abundance is regulated by viral infection and whether viral infection impacts cell cycle progression in a CDK2-dependent manner.
In fact, as illustrated in Figure 1, we investigated the changes in CDK2 at both the mRNA and protein levels following viral infection. Our findings revealed that SVCV infection resulted in an increase in CDK2 mRNA and protein expression. Additionally, our earlier reports have indicated that SVCV infection can induce alterations in the cell cycle, resulting in a notable increase in the S phase (Figure 1 of ref. 1). However, whether SVCV infection impacts cell cycle progression in a CDK2dependent manner will be explored in our upcoming study.
Reference:
(1) Li S, et al. Spring viraemia of carp virus modulates p53 expression using two distinct mechanisms. PLoS Pathog 15, e1007695 (2019).
(3) Provide data/discussion concerning the role of fish CDK2 in the regulation of cell cycle progression and whether this process is impacted by viral infection (part 1). Are TBK1 abundance and interferon production differentially regulated across the cell cycle due to the action of CDK2 (part 2).
Thank you for your advice. This concern is addressed in two parts, as follows:
For part 1: To date, there has been limited research conducted on fish CDK2 in the regulation of cell cycle progression. The details are as follows: It has been reported that the kinase activity of goldfish CDK2 significantly increases during oocyte maturation (ref. 1). Furthermore, UHRF1 phosphorylation by cyclin A2/CDK2 is crucial for zebrafish embryogenesis (ref. 2). Additionally, a novel CDK2 homolog has been identified in Japanese lamprey, which plays a crucial role in apoptosis (ref. 3). Red grouper nervous necrosis virus (RGNNV) infection activates the p53 pathway, leading to the upregulation of p21 and downregulation of cyclin E and CDK2, which forces infected cells to remain in the G1/S replicative phase (ref. 4). All this evidence suggests that fish CDK2 plays a vital role in cell cycle regulation, and this process is also impacted by viral infection. Relevant content has been added to the Discussion section in the revised manuscript (lines 389-398).
References:
(1) Hirai T, et al. (1992) Isolation and Characterization of Goldfish Cdk2, a Cognate Variant of the Cell-Cycle Regulator Cdc2. Developmental biology 152(1):113-120.
(2) Chu J, et al. (2012) UHRF1 phosphorylation by cyclin A2/cyclin-dependent kinase 2 is required for zebrafish embryogenesis. Molecular biology of the cell 23(1):5970.
(3) Xu Y, Tian Y, Zhao H, Zheng N, Ren KX, Li QW. A novel CDK-2 homolog identified in lamprey, with roles in apoptosis. Fish Physiol Biochem 47, 189-189 (2021).
(4) Mai WJ, Liu HX, Chen HQ, Zhou YJ, & Chen Y (2018) RGNNV-induced cell cycle arrest at G1/S phase enhanced viral replication via p53-dependent pathway in GS cells. Virus Res 256:142-152.
For part 2: TBK1 plays a crucial role in regulating IFN production. Variations in CDK2 activity during different phases of the cell cycle may lead to changes in the expression and function of TBK1. Our findings suggest that heightened CDK2 activity may suppress TBK1 expression, thereby hindering the cell's capacity to produce IFN. Conversely, during the late phase of the cell cycle or in an inhibited state, TBK1 expression may rise, enhancing IFN synthesis and release. In summary, CDK2 is involved in intracellular signaling by modulating TBK1 levels and IFN production, affecting the cellular immune response and cycle regulation—two processes that are notably distinct at various stages of the cell cycle. Relevant content has been added to the Discussion section in the revised manuscript (lines 377-384).
Minor suggestions:
(1) The authors introduce their study with the consideration that knowledge of fish signaling pathways can inform mammalian biology because mammals evolved from fish. This is not strictly true, since mammals and fish both evolved from an ancient common ancestor and the diversification of signaling in each species likely occurred in response to distinct evolutionary selective pressures.
Thank you for your suggestion. We have revised the statement in the manuscript to eliminate the notion that mammals evolved from fish (lines 98-99). The immune systems of higher vertebrates (e.g., humans) and lower vertebrates (e.g., fish) generally exhibit some consistency, although there are notable differences.
(2) On line 210 and line 276, the authors appear to have misstated the data. CDK2 knockout increases not decreases TBK1 and Dtx4 knockdown abrogated rather than restored CDK2 suppression of TBK1.
Thanks for your reminder, I jumped to the wrong conclusions in these two places (line 204 and line 267) and have changed them as you suggested.
Reviewer #2 (Recommendations For The Authors):
The manuscript has some shortcomings that, if addressed, could improve the overall quality of the article.
(1) Line 63-72, line 77-79, line 88-90- please add additional references for these sentences.
Thanks to your comment, we have added references for these sentences (Line 63-72, line 77-79, line 88-90).
(2) It is of the utmost importance to quantify the data presented in Figures 4J and 5D, as this will facilitate the visualization of the immunoblot.
Thank you for your comment. We have quantified the data presented in Figures 4J and 5D to enhance the clarity of the immunoblot.
(3) The scale in Figure 4E is difficult to discern.
Thanks for your comment. To improve the visual clarity of the image, we have enlarged the scale label in Figure 4E.
(4) In Figure 3B, shCDK2 is shown in italics, preferably in line with other standards such as Figures 3C and 3F.
Thank you for your comment. We have revised the shCDK2 in Figure 3B.
(5) The functions of CDK family members in immunity are hoped to be discussed.
Thanks for your suggestion. We have discussed the functions of CDK family members in immunity (lines 363-387). The details are as follows: Recent studies have demonstrated that CDK activity is crucial for virus-induced innate immune responses. Reports indicate that CDKs are involved in the Toll-like receptor (TLR) signaling pathway, the nuclear factor-κB (NF-κB) signaling pathway, and the JAK-STAT signaling pathway. For instance, CDK8 and/or CDK19 enhanced the transcription of inflammatory genes, such as IL-8 and IL-10, in cells following TLR9 stimulation. CDKs and NF-κB establish a remarkable paradigm where CDKs can act directly on substrate proteins rather than depending solely on transcriptional control. It has been reported that CDK1 serves as a positive regulator of the IFN-I signaling pathway, facilitating STAT1 phosphorylation, which subsequently boosts the expression of ISGs. Furthermore, inhibiting CDK activity has been shown to obstruct STAT phosphorylation, proinflammatory gene activation, and ISG mRNA induction in response to SeV infection. It is important to note that no evidence suggests the involvement of CDKs in RLR signaling pathways. This study has shown that fish CDK2 functions as a negative regulator of the key kinase TBK1, which is involved in the RLR signaling pathway. A better understanding of the relationship between CDK2 and RLR signaling pathways will enhance our grasp of the regulatory mechanisms of CDKs in antiviral innate immunity.







