Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorLarissa CunhaUniversity of Sao Paulo, Ribeirão Preto, Brazil
- Senior EditorTadatsugu TaniguchiThe University of Tokyo, Tokyo, Japan
Reviewer #1 (Public review):
Summary:
The authors set out to evaluate the regulation of interferon (IFN) gene expression in fish, using mainly zebrafish as a model system. Similar to more widely characterized mammalian systems, fish IFN is induced during viral infection through the action of the transcription factor IRF3 which is activated by phosphorylation by the kinase TBK1. It has been previously shown in many systems that TBK1 is subjected to both positive and negative regulation to control IFN production. In this work, the authors find that the cell cycle kinase CDK2 functions as a TBK1 inhibitor by decreasing its abundance through recruitment of the ubiquitinylation ligase, Dtx4, which has been similarly implicated in the regulation of mammalian TBK1. Experimental data are presented showing that CDK2 interacts with both TBK1 and Dtx4, leading to TBK1 K48 ubiqutinylation on K567 and its subsequent degradation by the proteasome.
Strengths:
The strengths of this manuscript are its novel demonstration of the involvement of CDK2 in a process in fish that is controlled by different factors in other vertebrates and its clear and supportive experimental data.
Weaknesses:
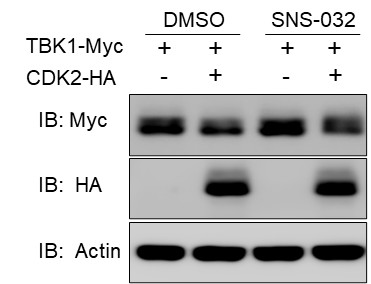
The weaknesses of the study include the following. 1) It remains unclear how CDK is regulated during viral infection and how it specifically recruits E3 ligase to TBK1. The authors find that its abundance increases during viral infection, an unusual finding given that CDK2 levels are often found to be stable. How this change in abundance might affect cell cycle control was not explored. 2) The implications and mechanisms for a relationship between the cell cycle and IFN production will be a fascinating topic for future studies. In particular, it will be critical to determine if CDK2 catalytic activity is required. An experiment with an inhibitor suggests that this novel action of CDK2 is kinase independent, but the lack of controls showing the efficacy of the inhibitor prevents a firm conclusion. It will also be critical to determine if there is a role for cyclins in this process or if there is competition for binding between TBK1 and cyclin and, if so, if this has an impact on the cell cycle. Likewise, an impact of CDK2 induction by virus infection on normal cell cycling will be important to investigate.
Reviewer #2 (Public review):
Summary:
In this paper, the authors describe a novel function involving the cell cycle protein kinase CDK2, which binds to TBK1 (an essential component of the innate immune response) leading to its degradation in a ubiquitin/proteasome-dependent manner. Moreover, the E3 ubiquitin ligase, Dtx4, is implicated in the process by which CDK2 increases the K48-linked ubiquitination of TBK1. This paper presents intriguing findings on the function of CDK2 in lower vertebrates, particularly its regulation of IFN expression and antiviral immunity.
Strengths:
(1) The research employs a variety of experimental approaches to address a single question. The data are largely convincing and appear to be well executed.
(2) The evidence is strong and includes a combination of in vivo and in vitro experiments, including knockout models, protein interaction studies, and ubiquitination analyses.
(3) This study significantly impacts the field of immunology and virology, particularly concerning the antiviral mechanisms in lower vertebrates. The findings provide new insights into the regulation of IFN expression and the broader role of CDK2 in immune responses. The methods and data presented in this paper are highly valuable for the scientific community, offering new avenues for research into antiviral strategies and the development of therapeutic interventions targeting CDK2 and its associated pathways.