Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorRafael Fernández-ChaconInstituto de Biomedicina de Sevilla (IBiS, HUVR/CSIC/Universidad de Sevilla), Sevilla, Spain
- Senior EditorDavid RonUniversity of Cambridge, Cambridge, United Kingdom
Reviewer #1 (Public Review):
Mitochondria are essential organelles consisting in mammalian cells of about 1500 different proteins. Most of those are synthesized in the cytosol as precursor proteins, imported into mitochondria, and sorted into one of the four sub-mitochondrial compartments. The TIM23 complex, which is embedded in the mitochondrial inner membrane, facilitates the import of proteins that harbor Mitochondrial Targeting Sequence (MTS) at their N-terminus. Such proteins are sorted mainly to the mitochondrial matrix while some sub-groups are destined also to the inner membrane or the intermembrane space. TIMM50 (Tim50 in yeast) is an essential component of the TIM23 complex and mutations in this protein were reported to cause several diseases.
Summary:
In the current study, the authors analyzed the impact of TIMM50 mutations on the mitochondrial proteome in both patients' cells and mouse neurons. They provide compelling evidence for several surprising and highly interesting observations: (i) TIMM50 mutations affect the steady-state levels of only a portion of the putative TIMM50 substrates, (ii) such mutations result in increased electrical activity in mice neurons and in reduced levels of some potassium ion channels in the plasma membrane. These findings shed new light on mitochondrial biogenesis in mammalian cells and hint at an unexpected link between mitochondria and ion channels at the plasma membrane.
Strengths:
The authors used both cells from patients and neurons from mice to investigate the impact of mutations in TIMM50 on mitochondrial proteome and function.
Weaknesses:
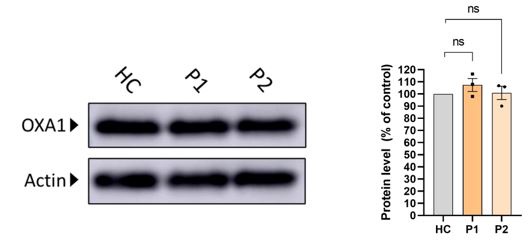
(1) It will be interesting to monitor the levels of another MIM insertase namely, OXA1. This will help to understand whether some of the observed changes in levels of OXPHOS subunits are related to alterations in the amounts of this insertase.
(2) The authors did not provide explanations for several key findings like:
A. Figure 3: How do the authors explain that although TIMM17 and TIMM23 were found to be significantly reduced by Western analysis they were not detected as such by the Mass Spec. method?
B. How do the authors explain the higher levels of some proteins in the TIMM50 mutated cells?
C. Can the authors elaborate on why mutated cells are impaired in their ability to switch their energetic emphasis to glycolysis when needed?
Reviewer #2 (Public Review):
Summary:
Mitochondria import hundreds of precursor proteins from the cytosol. The TOM and TIM23 complexes facilitate the import of the matrix-targeting pathway of mitochondria. In yeast, Tim50 is a critical and essential subunit of the TIM23 complex that mediates the transition of precursors from the outer to the inner membrane. The human Tim50 homolog TIMM50 is highly similar in structure and a comparable function of Tim50 and TIMM50 was proven by several biochemical and genetic studies in the past.
In this study, the authors characterize human cells that express lower levels or mutated versions of TIMM50. They found that in these TIMM50-depletion cells, the levels of other TIM23 core subunits are also diminished but many mitochondrial proteins are unaffected. Moreover, they observed alterations in the electrical activity and the levels of potassium channels in neuronal cells of TIMM50-deficient mice. They propose that these changes explain the pathology of patients who often suffer from epilepsy.
Strengths:
The paper is written by experts in the field, and it is very clear. The experiments are of high quality and sufficiently well-controlled. The study is interesting for a broad readership.
Weaknesses:
However, there is a problem that needs to be fixed: The interpretation of the results needs to be downgraded. The authors claim in the abstract, the introduction, and the discussion that TIMM50 and the TIM23 translocase might not be relevant for mitochondrial protein import in mammals. This is misleading and certainly wrong!!! What they do show is that the depletion of TIMM50 to about 20% of the normal levels is still tolerated in cultured cells grown in a glucose-rich medium. This is similar to the situation of cytochrome oxidase deficiency where lower levels are often well tolerated, and only very low levels (<20%) lead to Leigh syndrome. Still, it would be wrong to claim that respiration can occur without the terminal enzyme. Thus, it is essential that the authors change the wording here. In the discussion they offer several explanations: Mitochondria might import more proteins than they need and just degrade the excess. The TIMM50 depletion cells might increase the expression of mitochondrial proteins as a compensatory mechanism. Translocation across the TIM23 complex might not be the rate-limiting step in mitochondrial protein biogenesis. The authors do not have to sort this out on a molecular level, however, they have to be more cautious with the conclusions they make! Except for this issue, this is a very interesting study that will raise the interest of the mitochondrial community.