UNC93B1 mediates differential trafficking of endosomal TLRs
Figures
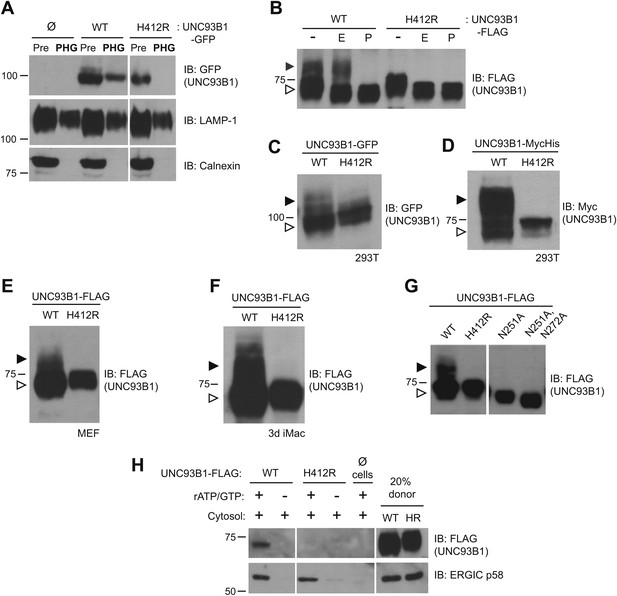
UNC93B1 traffics to the Golgi en route to endolysosomes.
(A) UNC93B1 is present in phagolysosomes of unstimulated cells. Phagosomes (PHG) isolated by flotation from RAW264 cells only (Ø) or expressing GFP tagged UNC93B1-WT or UNC93B1-H412R and cells prior to isolation (Pre) were separated by SDS-PAGE, and immunoblotted with anti-GFP, anti-LAMP1 (lysosome marker), and anti-calnexin (ER marker). (B) A portion of UNC93B1 protein traffics to the Golgi apparatus. Wildtype UNC93B1 (WT) or H412R, each with a C-terminal 3× FLAG tag, were expressed in HEK293Ts by transient transfection. The immunoprecipitated proteins were treated with EndoH (E), PNGaseF (P) or left untreated (−), separated by SDS-PAGE, and visualized by immunoblot with anti-FLAG antibody. Bands representing EndoH-sensitive (white arrow) and resistant (black arrow) forms of UNC93B1 are indicated. (C)–(F) UNC93B1 acquires EndoH-resistant modifications. UNC93B1 tagged with GFP (C) or myc-His (D) from transiently transfected HEK293Ts, and FLAG tagged UNC93B1 expressed in MEFs (E) or 3d iMac cells (F) were analyzed for the presence of EndoH-resistant glycans. Lysates were separated by SDS-PAGE and immunoblotted with the indicated antibodies. EndoH-sensitive (white arrow) and EndoH-resistant (black arrow) forms are indicated. (G) Mutation of UNC93B1 glycosylation sites abolishes EndoH resistant forms. Lysates from HEK293Ts transiently transfected with FLAG tagged UNC93B1-WT, -N251A or -N251A/N272A were separated by SDS-PAGE and immunoblotted with anti-FLAG antibody. (H) UNC93B1 is loaded into COPII vesicles. Digitonin-permeabilized COS7 cells expressing 3× FLAG-tagged UNC93B1-WT or UNC93B1-H412R, or no cells (Ø) were incubated with ATP regenerating system, GTP, and rat liver cytosol, as indicated, in an in vitro COPII budding assay. Vesicles purified by ultracentrifugation were analyzed by SDS-PAGE and immunoblot using the indicated antibodies. 20% of the COS7 cells prior to the budding reaction serves as a loading control (20% donor). ERGIC/p58 serves as a positive control for the formation of COPII vesicles. Results are representative of at least three experiments (A–G) or two experiments (H).
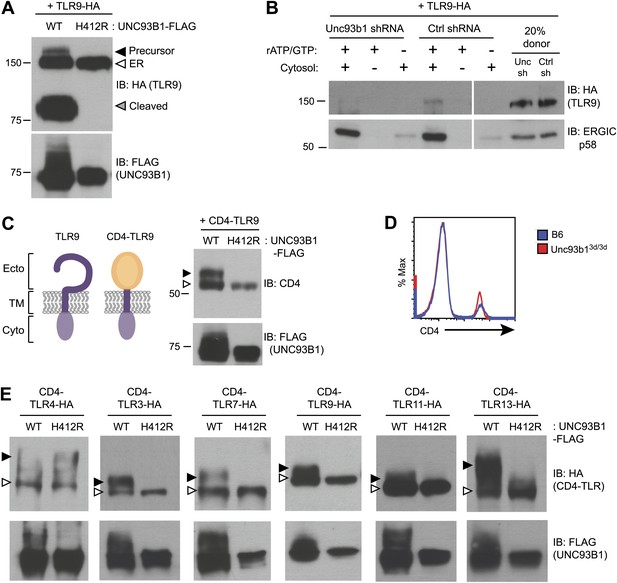
UNC93B1 controls ER exit of TLRs 3, 7, 9, 11, and 13.
(A) TLR9 fails to exit the ER in cells lacking functional UNC93B1. Lysates from 3d iMac cells complemented with either UNC93B1-WT or UNC93B1-H412R and expressing TLR9-HA were analyzed by SDS-PAGE and immunoblotted with the indicated antibodies. The precursor (black arrow), ER (white arrow) and cleaved (grey arrow) forms of TLR9-HA are indicated. (B) UNC93B1 is required for TLR9 loading into COPII vesicles. RAW264 macrophages stably transduced with retroviruses encoding control or Unc93b1-directed shRNA and expressing TLR9-HA were used in an in vitro COPII budding assay as described in (Figure 1H). Lysates of purified vesicles or donor membranes were probed with the indicated antibodies. (C) The transmembrane and cytosolic domain of TLR9 is sufficient to confer UNC93B1-dependence. (Left) schematic of TLR9 and the CD4-TLR9 chimera. Transmembrane (TM), ectodomain (Ecto) and cytosolic domain (Cyto) are indicated. (Right) CD4-TLR9 was expressed in HEK293Ts together with FLAG-tagged UNC93B1-WT or UNC93B1-H412R. Total lysates were analyzed by SDS-PAGE and immunoblotted with anti-CD4 and anti-FLAG antibodies. EndoH-sensitive (white arrow) and resistant (black arrow) forms are indicated. (D) CD4 trafficking to the cell surface is normal in Unc93b13d/3d cells. Splenocytes from C57BL/6 (blue line) or Unc93b13d/3d (red line) mice were stained with anti-CD4 and analyzed by flow cytometry. (E) CD4-TLR chimeric proteins for each of the indicated TLRs were expressed in HEK293Ts together with FLAG-tagged UNC93B1-WT or UNC93B1-H412R. Lysates were separated by SDS-PAGE and visualized by immunoblot with anti-HA and anti-FLAG antibodies. EndoH-sensitive (white arrows) and resistant (black arrows) forms are indicated. The chimeras were constructed as shown in Figure 1E, except with the addition of a C-terminal HA tag. Results are representative of at least three experiments (A, C, and E) or two experiments (B and D).
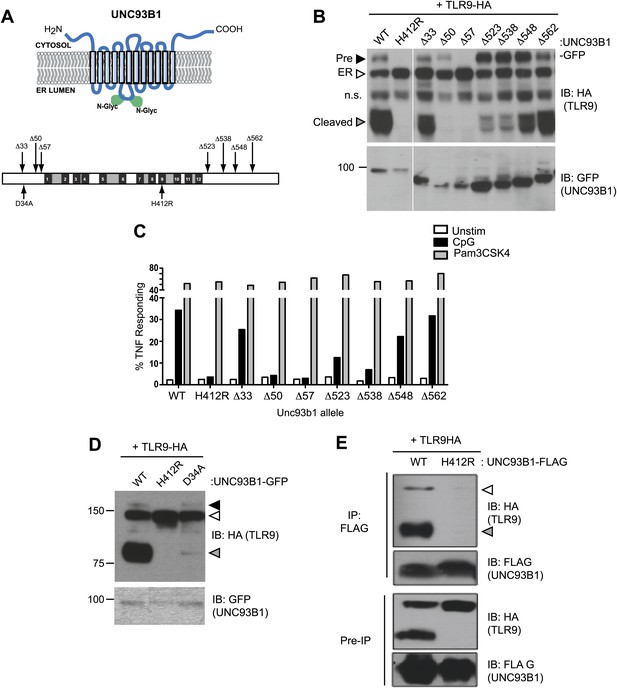
UNC93B1 mutants reveal two distinct roles in TLR9 trafficking.
(A) Schematic of predicted UNC93B1 topology. UNC93B1 is a 12 pass transmembrane protein with terminal regions facing cytosol. Two putative N-linked glycosylation (N-Glyc) sites are indicated (top). Schematic of UNC93B1 (1–598 a.a.). The black numbered boxes represent predicted transmembrane domains, white boxes represent regions predicted to face the cytosol, and grey boxes represent regions predicted to face the lumen. Truncation and point mutations are indicated with arrows (bottom). (B) N- and C-terminal mutants of UNC93B1 have distinct TLR9 trafficking outcomes. Lysates from 3d iMac cells expressing TLR9-HA and complemented with mutant forms of GFP-tagged UNC93B1 were subjected to SDS-PAGE and immunoblotted with anti-HA and anti-GFP antibodies. The precursor (black arrow), ER (white arrow) and cleaved (grey arrow) forms of TLR9-HA are indicated. n.s. indicates a non-specific band. (C) N- and C-terminal mutants of UNC93B1 have diminished TLR9 signaling. 3d iMac cells complemented with WT or indicated mutant alleles of UNC93B1 were harvested for intracellular TNFα staining 5 hr after stimulation with 3 μM CpG, 1 μg/ml Pam3CSK4 or left unstimulated. Percentages of TNF-producing cells after gating on UNC93B1-GFP positive cells are plotted. (D) TLR9 trafficking to endolysosomes is impaired in UNC93B1-D34A cells. 3d iMac cells were complemented with GFP-tagged UNC93B1-WT, -H412R or -D34A. Lysates were separated by SDS-PAGE and visualized by immunoblot with anti-HA and anti-GFP antibodies. The precursor (black arrow), ER (white arrow) and cleaved (grey arrow) forms of TLR9-HA are indicated. (E) UNC93B1 interacts with the cleaved form of TLR9. Immunoprecipitation of FLAG tagged UNC93B1 (WT or H412R) in 3d iMac cells expressing TLR9-HA was performed in 1% digitonin with anti-FLAG matrix. UNC93B1 associated proteins were analyzed by SDS-PAGE and immunoblot with anti-FLAG and anti-HA antibodies. ER (white arrows) and cleaved (grey arrows) forms of TLR9-HA are indicated. All results are representative of at least three experiments.
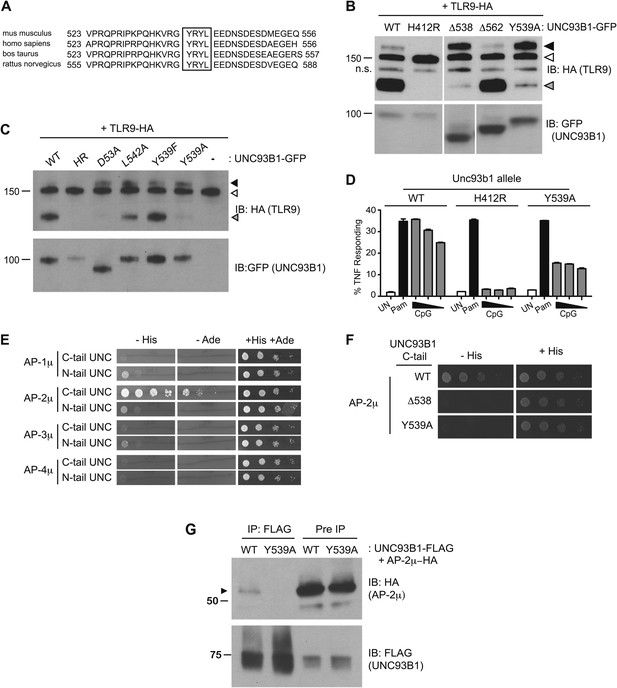
UNC93B1 controls post-Golgi trafficking of TLR9 by recruiting AP-2.
(A) Multi-species protein sequence alignment of residues in the UNC93B1 C-terminal tail. The YxxΦ motif, YRYL, is boxed. (B) Precursor TLR9 accumulates in UNC93B1-Y539A expressing cells. Lysates from 3d iMac cells expressing TLR9-HA were complemented with the indicated GFP tagged UNC93B1, analyzed by SDS-PAGE, and immunoblotted with anti-HA and anti-GFP antibodies. The precursor (black arrow), ER (white arrow) and cleaved (grey arrow) forms of TLR9-HA are indicated. n.s. indicates a non-specific band. (C) Mutations of YxxΦ in UNC93B1 confer partial phenotypes in TLR9 trafficking when compared with Y539A. Lysates from 3d iMac cells expressing TLR9-HA and GFP tagged UNC93B1-WT, H412R, Δ538, L542A, Y539F, Y539A were analyzed by SDS-PAGE and immunoblotted with anti-HA and anti-GFP antibodies. Presence of precursor (black arrow), ER (white arrow) and cleaved (grey arrow) forms of TLR9 are indicated. (D) TLR9 signaling is impaired in UNC93B1-Y539A cells. 3d iMac cells, complemented with GFP tagged UNC93B1-WT, -H412R, or -Y539A, were harvested for intracellular TNFα staining 5 hr after stimulation with 3 μM CpG, 1 μg/ml Pam3CSK4 (Pam) or left unstimulated. Percentages of TNF-producing cells after gating on UNC93B1-GFP positive cells are plotted. (E) The C-terminal tail of UNC93B1 interacts with AP-2μ. Results from a yeast two-hybrid assay testing for interaction between the AP (-1A, -2, -3A, -4) µ subunits and the N- or C-terminal cytosolic regions of UNC93B1 (N- or C-tail UNC), are shown. Growth on –His–Trp–Leu plates (–His) or –Ade–Trp–Leu (–Ade) indicates interaction. Growth on –Trp–Leu plates (+His+Ade) serves as a control. (F)–(G) Tyr-539 on UNC93B1 mediates interaction with AP-2 complex. (F) Results from a yeast two-hybrid assay testing for interaction between the AP-2µ subunit and the C-terminal cytosolic region of UNC93B1 (UNC93B1 C-tail) from WT, the Y539A mutant, or the Δ538 mutant are shown. Growth on –His–Trp–Leu plates (–His) indicates interaction. Growth on –Trp–Leu plates (+His) serves as a control. (G) HEK293T were transiently transfected with AP-2µ-HA and FLAG tagged UNC93B1-WT, -Y539A. Cell lysates were incubated with anti-FLAG matrix, and UNC93B1-associated proteins were eluted with FLAG peptide, separated by SDS-PAGE and visualized by immunoblot with anti-HA or anti-FLAG antibodies. UNC93B1 associated AP-2µ is indicated with black arrow. Results are representative of at least three experiments (B, D–F) or two experiments (C and G).
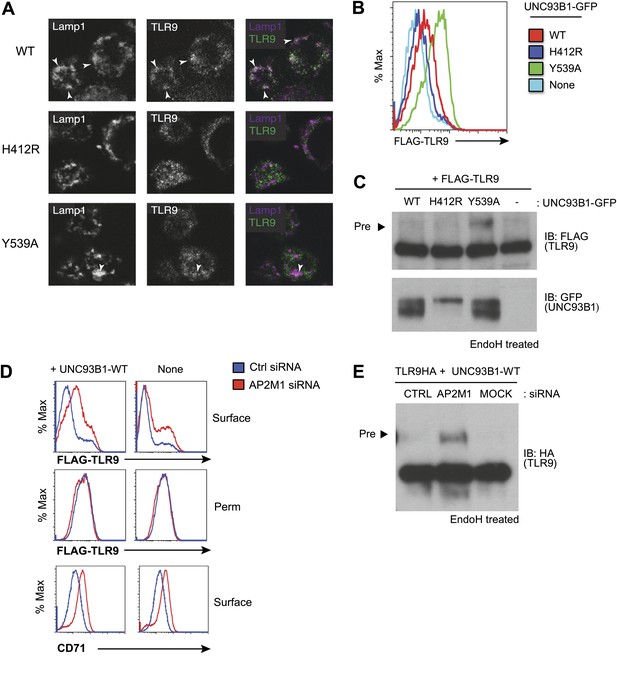
Failure to recruit AP-2 by UNC93B1 results in cell surface accumulation of TLR9.
(A) TLR9 fails to traffic to endolysosomes in UNC93B1-Y539A expressing cells. Localization of TLR9-HA and Lamp-1 in 3d iMac cells complemented with UNC93B1-WT, -H412R, or -Y539A was determined by immunofluorescence microscopy. Representative images of Lamp-1 (left), TLR9 (middle), and a pseudo-colored merged image for each UNC93B1 allele are shown. Arrowheads indicate areas of Lamp-1/TLR9 colocalization. (B) TLR9 accumulates at the cell surface in UNC93B1-Y539A expressing cells. HEK293Ts transfected with N-terminally tagged 3× FLAG-TLR9 and the indicated UNC93B1 alleles were stained with anti-FLAG and goat anti-mouse IgG secondary antibodies. FLAG staining was measured by flow cytometry. (C) The TLR9 precursor form accumulates in HEK293Ts expressing N-terminally tagged FLAG-TLR9 and UNC93B1-Y539A. Lysates from HEK293Ts stably expressing N-terminally tagged 3× FLAG TLR9 and GFP tagged UNC93B1-WT, -H412R, or -Y539A were harvested, treated with EndoH, separated by SDS-PAGE, and visualized by immunoblot with anti-FLAG and anti-GFP antibodies. (D) TLR9 accumulates at the cell surface in cells lacking AP-2. HEK293Ts stably expressing 3× FLAG-TLR9 with or without WT UNC93B1 were treated with AP-2μ (AP2M1) or control siRNA for 96 hr. FLAG staining was measured on intact (surface) or permeabilized (Perm) cells as described in (G). Anti-CD71 (transferrin receptor) staining serves as a control for AP-2 knockdown. (E) Absence of AP-2 causes accumulation of TLR9 precursor form. HEK293Ts were treated with control or AP-2µ siRNA. After 48 hr, siRNA treated cells were transiently transfected with TLR9-HA and GFP tagged UNC93B1-WT. Lysates were harvested 24 hr later for EndoH assay as described in (A). The TLR9 precursor form (black arrow) is indicated in each panel. Results are representative of at least three experiments (B and D) Or two (A, C, and E).
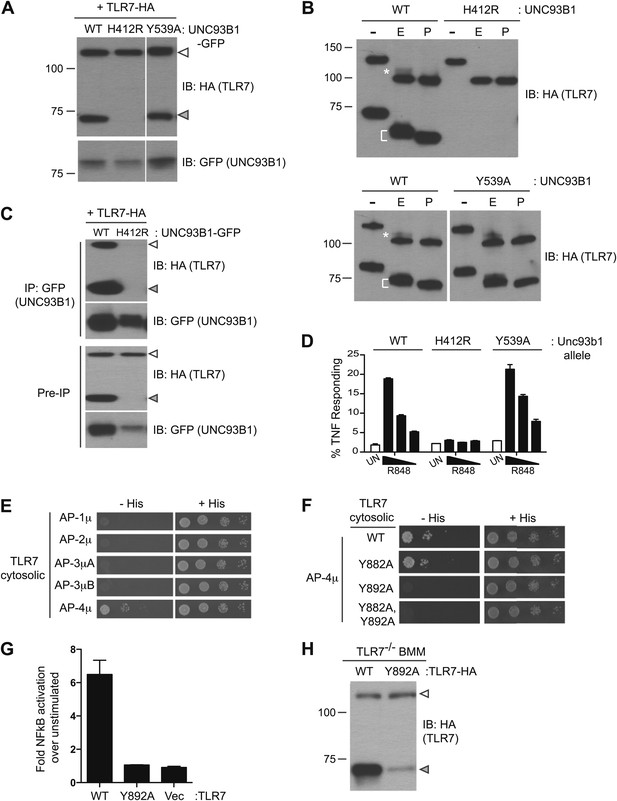
Differential trafficking of TLR7 and TLR9.
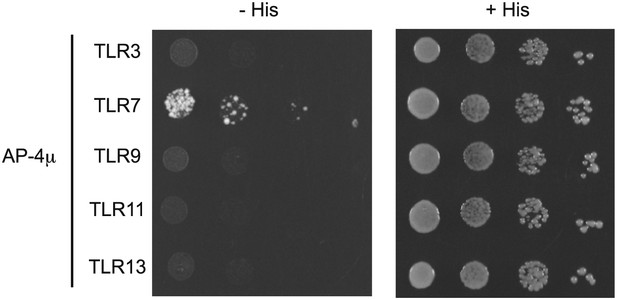
(A) TLR7 trafficking can be monitored biochemically and is UNC93B1-dependent. Lysates from 3d iMac cells expressing TLR7-HA and complemented with GFP-tagged UNC93B1-WT, -H412R or -Y539A were separated by SDS-PAGE and immunoblotted with anti-HA and anti-GFP antibodies. ER (white arrows) and cleaved (grey arrows) forms of TLR7-HA are indicated. (B) TLR7 acquires EndoH resistance. TLR7-HA was immunoprecipitated from 3d iMac cells expressing UNC93B1-WT, -H412R, or -Y539A, treated with EndoH (E), PNGaseF (P) or left untreated (−), and visualized by anti-HA immunoblot. Asterisk indicates precursor form of the receptor. The bracket indicates the migration difference between EndoH-treated and PNGaseF-treated TLR7. (C) UNC93B1 interacts with ER and cleaved forms of TLR7. GFP-tagged UNC93B1-WT or -H412R were immunoprecipitated from 3d iMac cells expressing TLR7-HA. UNC93B1 associated proteins were analyzed by SDS-PAGE and immunoblotted with anti-HA and anti-GFP antibodies. ER (white arrows) and cleaved (grey arrows) forms of TLR7-HA are indicated. (D) TLR7 signaling is normal in UNC93B1-Y539A cells. 3d iMac cells complemented with GFP tagged UNC93B1-WT, -H412R, or -Y539A were stimulated with 100 to 25 ng/ml R848 for 5 hr and intracellular TNFα stain was performed. Percentages of TNF-producing cells after gating on UNC93B1-GFP positive cells are plotted. (E) TLR7 interacts with AP-4μ. Results from a yeast two-hybrid assay testing for interaction between the AP-1, AP-2, AP-3, and AP-4 µ subunits and the C-terminal cytosolic region of TLR7. Growth on –His–Trp–Leu plates (–His) indicates interaction. Growth on –Trp–Leu plates (+His) serves as a control. (F) TLR7-Y892A is unable to interact with AP-4μ. Results from a yeast two-hybrid assay testing for interaction between the AP-4μ and TLR7 YxxΦ mutants (Y882A, Y892A, or double). Conditions are as described in (E). (G) TLR7-Y892A does not respond to TLR7 ligands. HEK293T cells were transiently transfected with an NF-κB luciferase reporter as well as expression plasmids encoding TLR7, TLR7-Y892A, or empty vector. Luciferase production was assayed 16 hr after stimulation with 10 µg/ml R848. (H) TLR7-Y892A trafficking is impaired. TLR7−/− bone marrow derived macrophages (BMMs) were transduced with HA-tagged TLR7-WT or TLR7-Y892A. Cell lysates were analyzed by SDS-PAGE and immunoblotted with anti-HA antibodies. ER (white arrows) and cleaved (grey arrows) forms of TLR7-HA are indicated. Results are representative of at least three experiments (A, D, and E) or two experiments (B, C, and F–H).
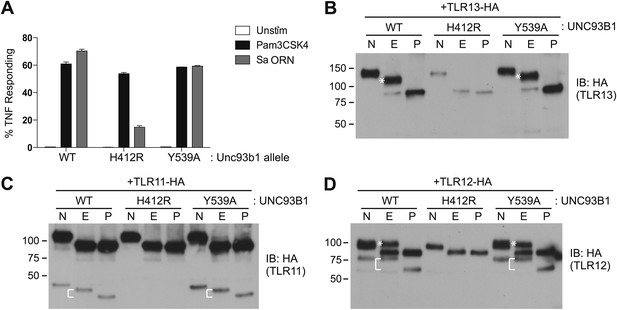
TLR11, TLR12, and TLR13 traffic independently of the UNC93B1/AP-2 pathway.
(A) TLR13 signaling is unaffected by UNC93B1-Y539A. 3d iMac cells expressing UNC93B1-WT, -H412R, or -Y539A were stimulated with 1 μg/ml Pam3CSK4 (black) or 1 nM Sa ORN complexed with DOTAP (grey) and harvested for intracellular TNFα staining 5 hr after stimulation. Percentages of TNF-producing cells are plotted. (B) TLR13 trafficking is unaffected by UNC93B1-Y539A. TLR13-HA was immunoprecipitated from 3d iMac cells expressing UNC93B1-WT, -H412R, or -Y539A, treated with EndoH (E), PNGaseF (P) or left untreated (N), and visualized by anti-HA immunoblot. Asterisk indicates full length EndoH resistant form of the receptor. (C) and (D) TLR11 (C) and TLR12 (D) trafficking is unaffected by UNC93B1-Y539A. TLR11 and TLR12 was treated as in (B). Asterisk indicates full length EndoH resistant form of the receptor. The bracket indicates the migration difference between EndoH-treated and PNGaseF-treated cleaved form of the receptors. Results are representative of at least three experiments (A) or two experiments (B–D).
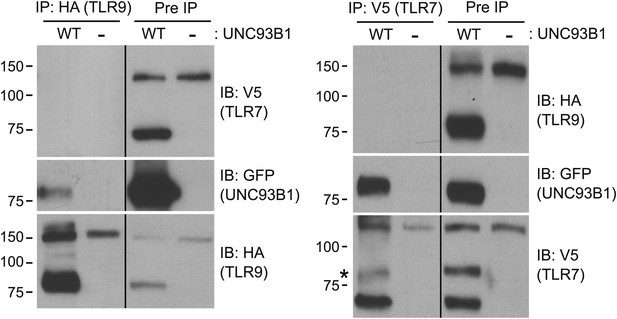
TLR7 and TLR9 association with UNC93B1 is mutually exclusive.
TLR9 and TLR7 associate with UNC93B1 in distinct complexes. 3d iMac cells expressing TLR9-HA, TLR7-V5-His, and WT UNC93B1-GFP (WT) or no UNC93B1 (−) were lysed in TNT buffer conditions then incubated with anti-HA matrix or anti-V5 protein A/G beads. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotted with HA, GFP and V5 antibodies. Asterisk indicates remaining UNC93B1-GFP signal. Results are representative of two experiments.
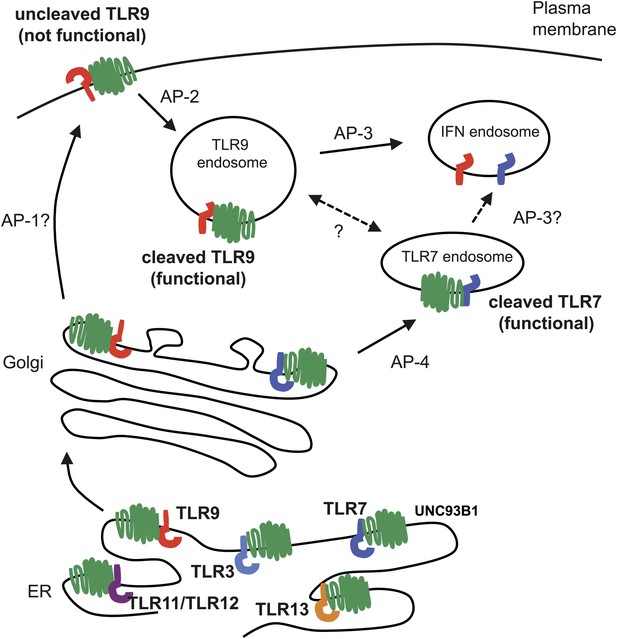
Trafficking pathways controlling localization of endosomal TLRs.
UNC93B1 interacts with several TLRs in the ER and facilitates loading into COPII vesicles. Unlike typical COPII loading factors, UNC93B1 remains associated with TLR9 and TLR7 after exit from the ER. Through its recruitment of AP-2, UNC93B1 is necessary for endocytosis of TLR9 from the plasma membrane into endosomes. TLR7 does not rely on this trafficking route. Instead, TLR7 utilizes AP-4 to bypass the cell surface and traffic directly to endosomes. This difference in trafficking may result in TLR9 and TLR7 accessing distinct compartments with unique functional properties related to the function of each receptor.