Neuroscience: ‘Cryptic’ exons reveal some of their secrets
When the DNA in the genes of a eukaryote has been transcribed into RNA, sequences of bases known as introns are then removed from the RNA transcript, and the remaining sequences, which are called exons, are spliced back together to produce messenger RNA. Most of the time this mRNA is then translated into a string of amino acids, which subsequently folds to form a protein. It has been known for some time that certain exons can be included or excluded when splicing together the mature mRNA. This form of splicing, which is known as alternative splicing, is generally considered to be a mechanism for allowing a single gene to code for two or more proteins (Nilsen and Graveley, 2010).
However, it is also known that alternative splicing can contribute to mechanisms that are used to control the abundances of certain proteins: in particular, mRNA degradation pathways in cells can target mRNA that has been spliced in a certain way. Now, writing in eLife, Taesun Eom and Robert Darnell of Rockefeller University, and co-workers at Rockefeller and Baylor College of Medicine, shed new light on these processes by identifying a previously unknown class of exons in RNA transcripts produced in neuronal cells (Eom et al., 2013). These ‘cryptic’ exons can be regulated by changes in neuronal activity, and might also help the brain to recover from seizures.
Over the years Darnell and co-workers have investigated the roles of RNA binding proteins in the nervous system. In particular, they have studied RNA binding proteins known as NOVA proteins and shown that they have a critical role in the cell nucleus, where they help to regulate alternative splicing (Ule et al., 2005). These NOVA proteins can also shuttle from the nucleus to the cytoplasm, and it is thought that they can modulate the abundances of proteins in neuronal cells by helping to localize RNA to dendrites (Racca et al., 2010).
To explore the differences between the nuclear and cytoplasmic roles of NOVA, Eom and colleagues separated brain tissue from mice into nuclear and cytoplasmic fractions, and employed a technique called HITS-CLIP (Licatalosi et al., 2008) to study the binding between the NOVA proteins and the RNA in these two fractions. They found that in the nucleus the NOVA proteins bind primarily to intronic regions of the RNA transcript: however, in the cytoplasm they bind mostly to 3′ untranslated regions of the transcripts. The Rockefeller-Baylor team then studied mice in which the genes for NOVA proteins had been knocked out, and found hundreds of RNA transcripts that were present at lower levels in these animals than in wild-type mice: they also found a smaller number of RNA transcripts that were present at higher levels in the knockout mice. Moreover, they found that NOVA proteins predominantly bind to these RNA transcripts in the nucleus, which was initially surprising because RNA binding proteins are generally thought to regulate mRNA levels by binding to the untranslated regions of RNA transcripts in the cytoplasm.
These results led Eom and co-workers to explore the intronic regions that the NOVA proteins bind to: they wanted to see if these regions contained exons that could be included in mRNA, and this is indeed what they found. In general these cryptic exons were found at low levels in the mature mRNA of wild-type mice, and at much higher levels in knockout mice (although a small number of RNA transcripts contain high numbers of cryptic exons in wild-type mice and low numbers in knockout mice). When included in RNA transcripts, these cryptic exons introduced premature translation stop codons into the mRNA. Instead of allowing full-length proteins to be produced, these codons trigger an mRNA degradation pathway called nonsense-mediated mRNA decay (Schoenberg and Maquat, 2012). The end result, therefore, is that cryptic exons can be used to influence the levels of proteins and mRNA in the cell (Figure 1).
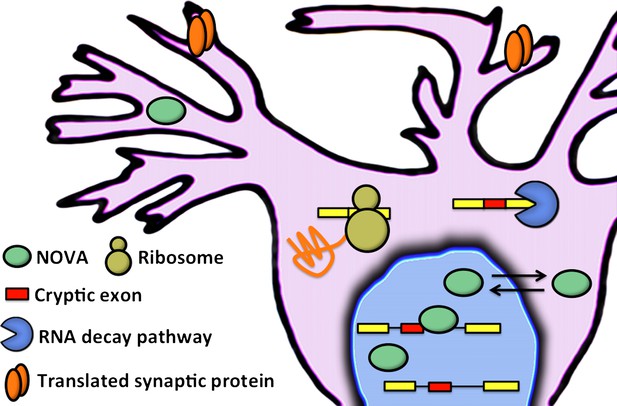
Eom and co-workers have shown that NOVA proteins (green ellipses) regulate the inclusion of cryptic exons (red boxes) in messenger RNA (mRNA) in the nucleus of neuronal cells. In the cytoplasm, mRNA that contains cryptic exons is targeted by RNA decay pathways (shown here by the blue pac-man), while mRNA that does not contain cryptic exons is translated by the ribosome (yellow) to produce synaptic proteins (orange). Neuronal activity can cause the NOVA proteins to shuttle from the nucleus to the cytoplasm: this changes the proportion of cryptic exons that are included in the mRNA, and therefore alters the abundance of the corresponding proteins.
In further experiments designed to explore the physiological relevance of this novel layer of NOVA-mediated gene regulation, the Rockefeller-Baylor team found that treating mice with a compound called pilocarpine, which modulates neuronal activity and induces seizures in animals, led to increased inclusion of certain cryptic exons. Intriguingly, in some regions of the brain, NOVA proteins move from the nucleus to the cytoplasm after treatment with pilocarpine. Moreover, mice that have lost one copy of the gene that codes for a specific NOVA protein (called NOVA 2) display spontaneous epileptic episodes. These results suggest that there is a connection between neuronal activity-dependent redistribution of NOVA proteins in the cell, the regulation of transcript levels by alternative splicing coupled to nonsense-mediated decay, and a requirement for NOVA proteins in maintaining balance in neuronal activity, potentially after excitatory stress.
This work is exciting for several reasons. First, the identification of a network of neuronal transcripts that is regulated by the coordinated action of alternative splicing and nonsense-mediated decay is a significant advance. It is well known that the coupling of these two gene regulatory layers has an important role in regulating the abundance of splicing factors, RNA binding proteins, and core components of the splicing machinery (Lareau et al., 2007; Saltzman et al., 2008). There have also been examples of individual splicing events in genes with important roles in neuronal development or function that are subject to this type of regulation (Boutz et al., 2007; Zheng et al., 2012). However, the set of transcripts identified in this latest study are all regulated in a coordinated manner by NOVA proteins, indicating that they likely contribute to aspects of neuronal biology as a module. In agreement with this idea, Eom and co-workers find that genes with NOVA-dependent cryptic exons often encode proteins enriched in similar functions at the synapse. A future goal will be to understand how this network of genes helps protect neurons from stress induced by neuronal activity. The results of such an analysis could yield new insights into and treatments for epilepsy.
Second, this work has also unmasked an important hidden layer of biology. The identification of cryptic exons regulated by NOVA would not have occurred without the use of HITS-CLIP and knockout mice because these exons are only present at very low levels (or are completely absent) in the transcripts of wild-type animals under normal behavioural conditions. These latest findings highlight the importance of performing more experiments on context-specific gene expression in dynamically regulated cells such as neurons. Future research involving RNA binding proteins other than NOVA proteins is sure to capture additional target transcripts subject to dynamic gene regulation in neurons. Indeed, several recent studies have demonstrated that this will likely be the case (Iijima et al., 2011; Yap et al., 2012), and provide an indication that there is much more uncharted RNA biology waiting to be decrypted.
References
-
The coupling of alternative splicing and nonsense-mediated mRNA decayAdv Exp Med Biol 623:190–211.
Article and author information
Author details
Publication history
Copyright
© 2013, Calarco
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,289
- views
-
- 173
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Neuroscience
Cancer patients often experience changes in mental health, prompting an exploration into whether nerves infiltrating tumors contribute to these alterations by impacting brain functions. Using a mouse model for head and neck cancer and neuronal tracing, we show that tumor-infiltrating nerves connect to distinct brain areas. The activation of this neuronal circuitry altered behaviors (decreased nest-building, increased latency to eat a cookie, and reduced wheel running). Tumor-infiltrating nociceptor neurons exhibited heightened calcium activity and brain regions receiving these neural projections showed elevated Fos as well as increased calcium responses compared to non-tumor-bearing counterparts. The genetic elimination of nociceptor neurons decreased brain Fos expression and mitigated the behavioral alterations induced by the presence of the tumor. While analgesic treatment restored nesting and cookie test behaviors, it did not fully restore voluntary wheel running indicating that pain is not the exclusive driver of such behavioral shifts. Unraveling the interaction between the tumor, infiltrating nerves, and the brain is pivotal to developing targeted interventions to alleviate the mental health burdens associated with cancer.
-
- Neuroscience
Emotional responsiveness in neonates, particularly their ability to discern vocal emotions, plays an evolutionarily adaptive role in human communication and adaptive behaviors. The developmental trajectory of emotional sensitivity in neonates is crucial for understanding the foundations of early social-emotional functioning. However, the precise onset of this sensitivity and its relationship with gestational age (GA) remain subjects of investigation. In a study involving 120 healthy neonates categorized into six groups based on their GA (ranging from 35 and 40 weeks), we explored their emotional responses to vocal stimuli. These stimuli encompassed disyllables with happy and neutral prosodies, alongside acoustically matched nonvocal control sounds. The assessments occurred during natural sleep states using the odd-ball paradigm and event-related potentials. The results reveal a distinct developmental change at 37 weeks GA, marking the point at which neonates exhibit heightened perceptual acuity for emotional vocal expressions. This newfound ability is substantiated by the presence of the mismatch response, akin to an initial form of adult mismatch negativity, elicited in response to positive emotional vocal prosody. Notably, this perceptual shift’s specificity becomes evident when no such discrimination is observed in acoustically matched control sounds. Neonates born before 37 weeks GA do not display this level of discrimination ability. This developmental change has important implications for our understanding of early social-emotional development, highlighting the role of gestational age in shaping early perceptual abilities. Moreover, while these findings introduce the potential for a valuable screening tool for conditions like autism, characterized by atypical social-emotional functions, it is important to note that the current data are not yet robust enough to fully support this application. This study makes a substantial contribution to the broader field of developmental neuroscience and holds promise for future research on early intervention in neurodevelopmental disorders.





