GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine
Figures
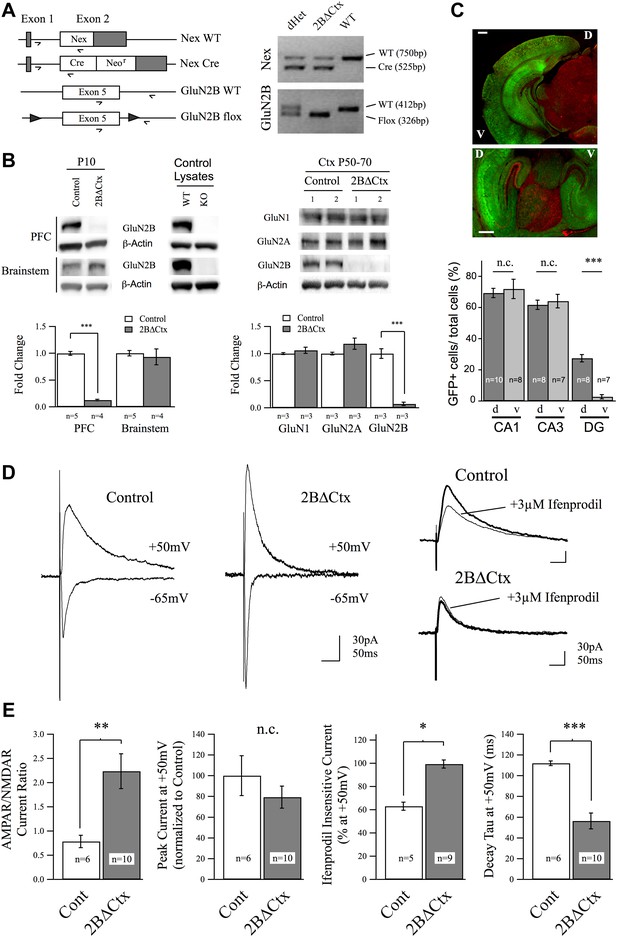
Genetic knockout of GluN2B from principal cortical neurons in vivo.
(A) Conditional ‘floxed’ GluN2B knockout mice were crossed with NEX-Cre animals to ablate GluN2B from principal cortical neurons in vivo (2BΔCtx). Genotyping strategy and data, generated using tail tissue DNA samples, are shown. (B) Western blots, normalized to actin, and quantification for cortical and brainstem lysates from animals at P10 demonstrate cortex-restricted suppression of GluN2B protein in 2BΔCtx mice (PFC = prefrontal cortex). Control lysates from P0 cortices of global GluN2B KO and WT animals run on the same blots served as positive and negative controls. Western blots and quantification of protein expression in control and 2BΔCtx cortex in vivo from P50–P70 animals shows selective decrease in GluN2B and no change in GluN1 and GluN2A. (C) Example images from NEX-Cre expressing DsRed flox-stop-flox GFP reporter mouse brain slices. Top, coronal section showing restricted cortical expression pattern of NEX-Cre (GFP+ = Cre+). Bottom, parasagittal slice showing strong Cre expression in both dorsal and ventral hippocampus and quantification, v = ventral, d = dorsal, scale bars = 500 µm. (D) Example traces recorded at +50 and −65 mV overlaid show the current response of layer II/III pyramidal neurons in control and 2BΔCtx slices from P18 to P21 animals in response to intracortical stimulation. Traces at +50 mV from control and 2BΔCtx slices demonstrate loss of ifenprodil sensitivity and faster decay kinetics of NMDAR-mediated current in 2BΔCtx neurons. (E) Combined analysis revealed a significant increase in the AMPAR to NMDAR-mediated current ratio in 2BΔCtx neurons, no change in overall peak current at +50 mV, a significant increase in the ifenprodil insensitive current at +50 mV, and a significant decrease in decay tau of the NMDAR-mediate current. Data values are means ± sem. *p < 0.05; **p < 0.01; ***p < 0.001 t test with respect to control; n.c. = no significant change.
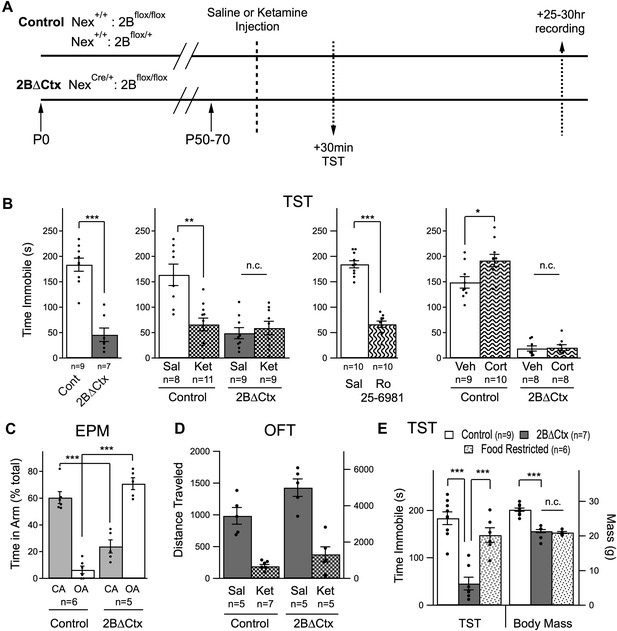
Decreased despair-like behavior and occlusion of ketamine's actions in 2BΔCtx animals.
(A) Experimental timeline: male animals between P50 and P70 were subjected to i.p. ketamine injection (ket) or saline control injection (sal). 30 min following injection, animals were analyzed in the tail suspension test (TST). At 25–30 hr post-injection, animals were subjected to electrophysiological analysis (see Figure 3). (B) A significant decrease in immobility scores was measured in 2BΔCtx animals in the TST and this was mimicked by treatment with the GluN2B-containing NMDAR selective antagonist Ro 25–6981. The decrease in immobility scores in the 2BΔCtx animals occluded effect of ketamine injection seen in littermate control animals. 2BΔCtx animals were also insensitive to chronic corticosterone treatment (Cort), while this same treatment increased immobility times in TST in control, corticosterone-treated animals. (C) 2BΔCtx exhibited a strong anxiolytic behavioral phenotype compared to control animals as measured in the elevated plus maze (EPM). (D) Measuring total distance traveled in the open field test (OFT) showed a strong and significant effect of ketamine in both genotypes, suggesting that the decreased immobility time in 2BΔCtx animals was not simply due to hyperlocomotion. (E) Food restriction was also used in control animals to demonstrate that the decreased immobility times in 2BΔCtx animals was not a consequence of decreased body mass. Data values are means ± sem. *p < 0.05; **p < 0.01; ***p < 0.001 t test with respect to control; n.c. = no significant change.
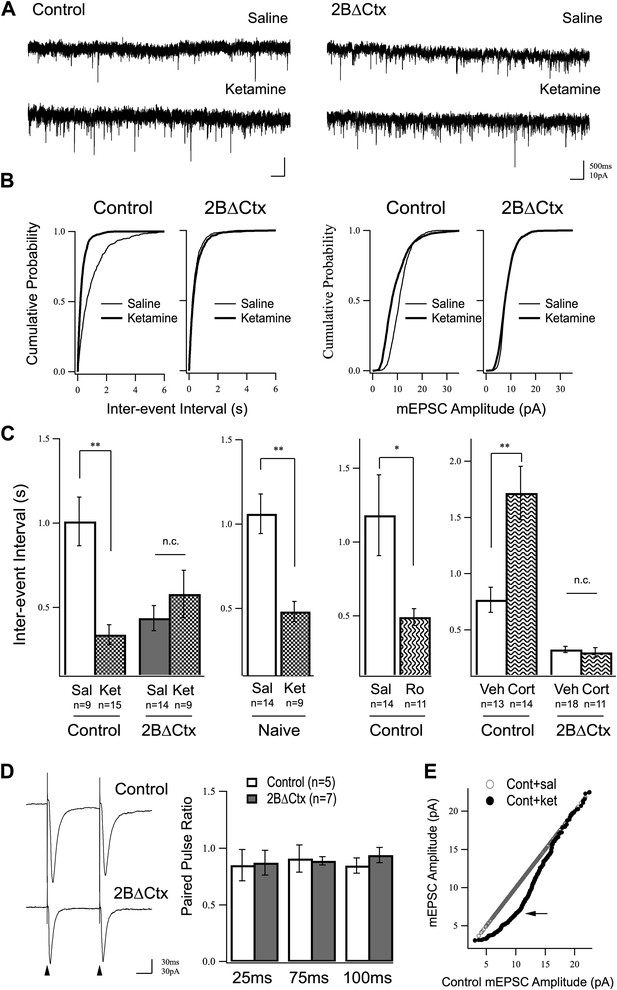
Increased excitatory synaptic transmission in prefrontal cortex following ketamine injection is occluded in 2BΔCtx animals.
(A) mEPSC recordings from prefrontal layer II/III cortical pyramidal neurons 24 hr after a single injection of saline (upper) or ketamine (lower) in control and 2BΔCtx mice. (B) Cumulative histograms showing the strong increase in frequency (decreased inter-event interval) (left) of mEPSC events in control animals by ketamine is occluded in 2BΔCtx littermates. (C) Quantification and statistical IEI results are shown for data in (B). The increase in mEPSC frequency was not due to subjecting the animals to the behavioral testing as ketamine significantly suppressed IEIs in behaviorally naïve control animals. Additionally, 2BΔCtx animals showed no change in frequency of events measured after chronic corticosterone treatment, while this caused a strong decrease in event frequency in control animals. (D) The increase in event number in 2BΔCtx prefrontal cortical neurons was not correlated with a change in the paired pulse ratio measured by evoking synaptic responses at this synapse (intracolumnar stimulation indicated by the arrowheads). Example traces of evoked responses recorded by whole-cell voltage clamp at 100 ms inter stimulus interval are shown. Quantification across a number of inter-stimulus intervals is presented. (E) Ranked mEPSC plot showing the disproportionate increase in small amplitude events (arrow) in control ketamine-injected animals compared to saline injected controls. Data values are means ± sem. *p < 0.05; **p < 0.01; t test with respect to control; n.c. = no significant change.
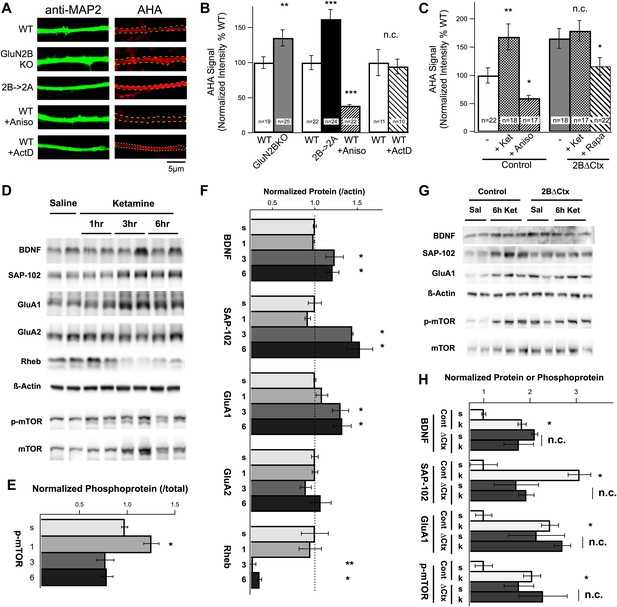
Changes in protein synthesis rates, synaptic protein expression, and phosphorylation in response to ketamine injection are occluded in GluN2B null neurons.
(A) FUNCAT was used to measure rates of protein synthesis in cortical neurons. AHA signal intensity in MAP2-stained dendrites reveals relative levels of new protein synthesized over a 6-hr period. Examples of 14 DIV cortical neurons from GluN2BKO and 2B→2A as well as WT control neurons treated with the protein synthesis inhibitor anisomycin (Aniso) or transcription inhibitor actinomycin-D (ActD). (B) Combined data show significant increase in protein synthesis rates in GluN2BKO and 2B→2A neurons and suppression of basal levels by anisomycin. (C) Combined data showing the significant increase in protein synthesis rates evoked by ketamine, occlusion of this increase in 2BΔCtx and sensitivity of the AHA signal to rapamycin in 2BΔCtx neurons. (D–H) Cortical synaptoneurosomes from animals following saline or ketamine injection. Western blot analysis showed the presence of mTOR in synaptoneurosomes as well as basal levels of phosphorylated protein (p-mTOR) and expression of BDNF, Rheb, and the synaptic proteins, SAP-102, GluA1, and GluA2. (D) Expression levels, and phosphorylation status of mTOR, following ketamine injection (1, 3, and 6 hr post injection) compared to saline-injected controls. (E) p-mTOR measured relative to total mTOR levels demonstrating time-dependent changes in phosphorylation status in response to ketamine. (F) Synaptic protein expression is shown in relation to levels of actin at 1, 3, or 6 hr post ketamine normalized to saline-injected controls. Increased levels of GluA1, SAP102, and BDNF as well as decreased rheb expression seen at 3 and 6 hr post injection. (G) Example of western blots at 6 hr post-injection for both control and 2BΔCtx genotypes. (H) Quantification of the data showed significant increases in synaptic proteins in response to ketamine (k), consistent with (A–C), but also revealing occlusion of these increases in SAP-102, GluA1 and p-mTOR in 2BΔCtx animals. Data values are means ± sem. **p < 0.01; ***p < 0.001 t test with respect to WT; n.c. = no significant change.
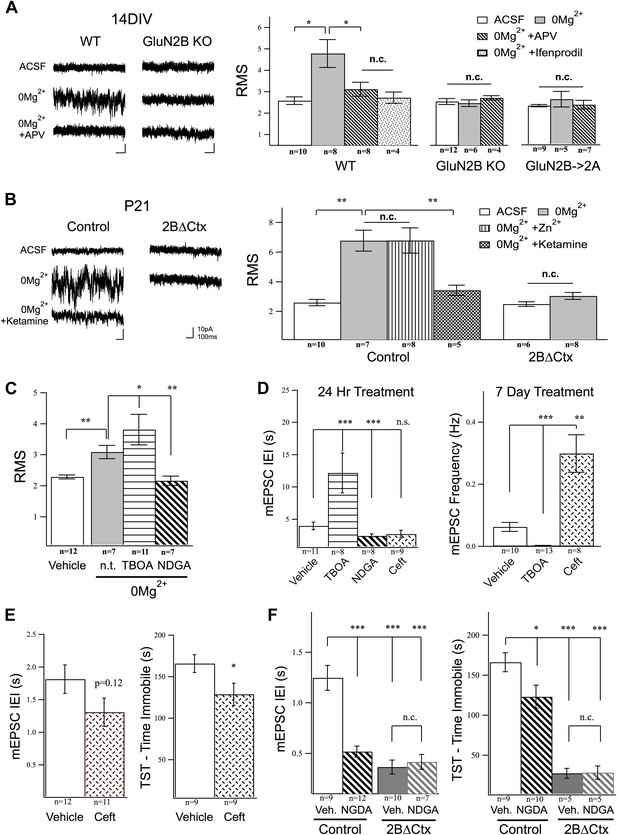
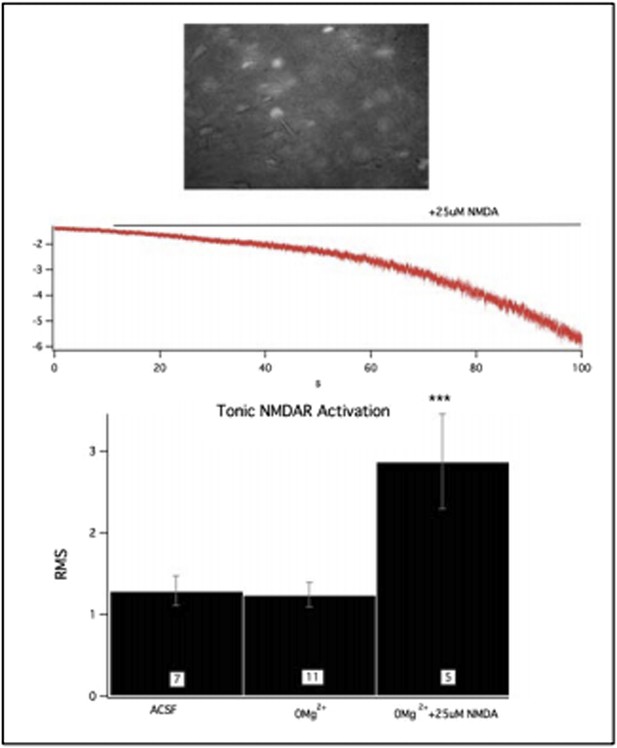
Activation of GluN2B-containing NMDARs by ambient glutamate regulates mEPSC frequency and depression-like behavior.
Whole-cell recordings of cortical pyramidal neurons (Vhold −65 mV) under control conditions (ACSF) and in 0Mg2+ ACSF were used to test the subunit contribution of the NMDA receptor pool activated by ambient glutamate. (A) Perfusion of 0Mg2+ ACSF uncovered a tonic current in cultured cortical neurons (14 DIV) evidenced by an increase in baseline current noise that could be reversed by the NMDAR antagonist APV. This tonic NMDAR-mediated current was completely absent in GluN2B null neurons, including those in which GluN2B had been genetically replaced by GluN2A (GluN2B→2A). (B) Ambient glutamate also evoked a tonic current in acute brains slices that was suppressed by ketamine and was absent in 2BΔCtx slices (P21). (C–D) Manipulation of tonic activation of GluN2B-containing NMDARs alters mEPSC frequency in cortical cultures and pyramidal neurons in vivo, as well as expression of depression-like behavior. (C) Acute treatment of cultures with the glutamate transporter antagonist dl-TBOA enhanced the evoked current, while NDGA, which enhances glutamate transporter function, decreased this current. (D) Elevating ambient glutamate by blocking transporters with dl-TBOA, or decreasing it by enhancing glutamate transporter function with NDGA, or upregulating glutamate transporter expression using ceftriaxone bidirectionally regulated mEPSC frequency in cultured cortical neurons. (E) Enhancing glutamate transporter expression by i.p. injection of ceftriaxone resulted in a decrease in mEPSC IEI, and significant antidepressant-like action in control mice. (F) Injections of NDGA resulted in a significant decrease in mEPSC IEI in pyramidal neurons and decreased immobility in the TST. The effect of NDGA was mimicked and occluded on both measures in 2BΔCtx mice. Data points are mean ± sem. p-values are *<0.05, **<0.01, ***<0.001, and n values are shown for each experiment. n.c. = no significant change.
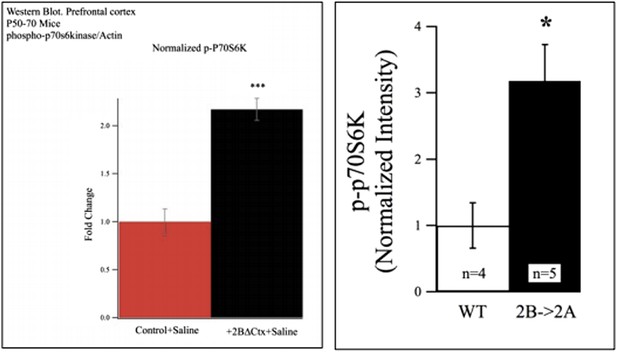
Western blot data from P50-P70 PFC showing increased p-P70S6K signal in lysates from 2BdeltaCTX animals (left) and 2B->2A animals (right).