Cis-interactions between Notch and its ligands block ligand-independent Notch activity
Figures
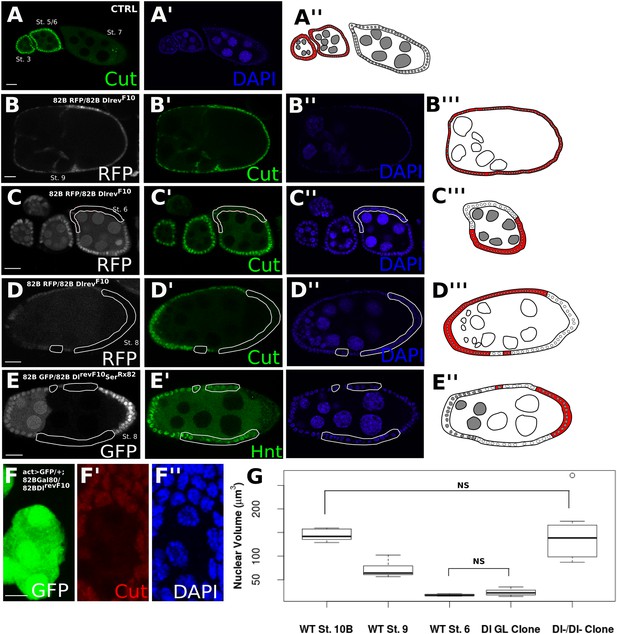
Follicle cells without DSL ligand bordering germline cells without DSL ligand show proper Notch activation and downstream differentiation.
Illustrations legend: active Notch = white cytoplasm, inactive Notch = red cytoplasm, WT cell = grey nuclei, mutant clone = white nuclei. (A–E). Follicle cells downregulate Cut at stage 7 of oogenesis (A). DlrevF10 mutant germline cells cause late Cut expression in follicle cells (B). DlrevF10 mutant follicle cells downregulate Cut early (C). DlrevF10 follicle cell clones bordering DlrevF10 germline clones show proper Cut downregulation (D). DlrevF10SerRx82 mutant follicle cell clones bordering DlrevF10SerRx82 germline clones also show proper Hnt (E). See Figure 1—figure supplement 2 for a z-series image for 1D and 1E. These germline/follicle cell clones (D and E) show increased nuclear size comparable to wild-type (WT) follicle cells which have entered the endocycle (n = 8 for each stage/genotype) (F and G). For (G), Welch t-tests were done to assess significance between each condition. The only comparisons that were not significant were between WT stage 10B and Dl-/Dl- clones and between WT stage 6 and Dl germline clones, indicating nuclear size in germline clones alone is similar to that of cells before the endocycle, whereas Dl-/Dl- clonal nuclei are more similar in size to cells that have entered the endocycle. Scale bars represent 20 μm, except in F, where the scale bar represents 5 μm.
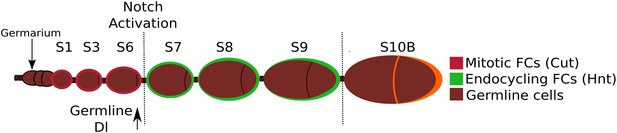
A schematic depiction of the early stages of Drosophila oogenesis.
Oogenesis begins in the germarium, where germline stem cells divide four times, producing a 16-cell germline cyst which is encapsulated by somatic follicle cells (FCs). When the FCs complete encapsulation and bud from the germarium, this is termed a stage 1 egg chamber. The egg chamber then grows and the FCs undergo mitosis until stage 6, and during these stages Cut is expressed and cells remain diploid. At stage 5, Dl is strongly upregulated in the germline. The transition from stage 6 to stage 7 is defined by activation of Notch, upregulation of Hnt, repression of Cut, and the endocycling of the FCs.
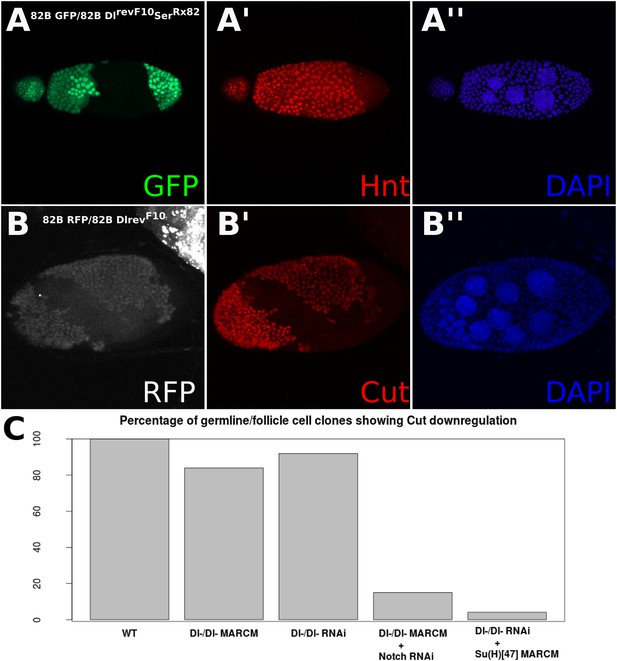
Z-stacked images of Dl-/Dl- clones and quantification of Cut staining in egg chamber clones.
Z series confocal images of DlrevF10SerRx82 (A) or DlrevF10 (B) germline/follicle cell clones from Figure 1D,E stained for Hnt (A) or Cut (B). Notice Hnt staining in the anterior end of (A) is owing to the formation of a partial germline clone containing both wild-type (WT; anterior, left) and DlrevF10SerRx82 (posterior, right) nurse cells, and therefore the anterior-most WT follicle cells have a ligand source to induce normal Hnt expression. Quantification of Cut expression in Dl-/Dl- clones induced by RNAi or by DlrevF10 homozygous mutant cells, and the effect of loss of Notch or Su(H) (C) (n = 30 for Dl-/Dl- MARCM, n = 25 for Dl-/Dl- RNAi, n = 38 for Dl-/Dl- MARCM + N RNAi, and n = 24 for Dl-/Dl- RNAi + Su(H)47 MARCM).
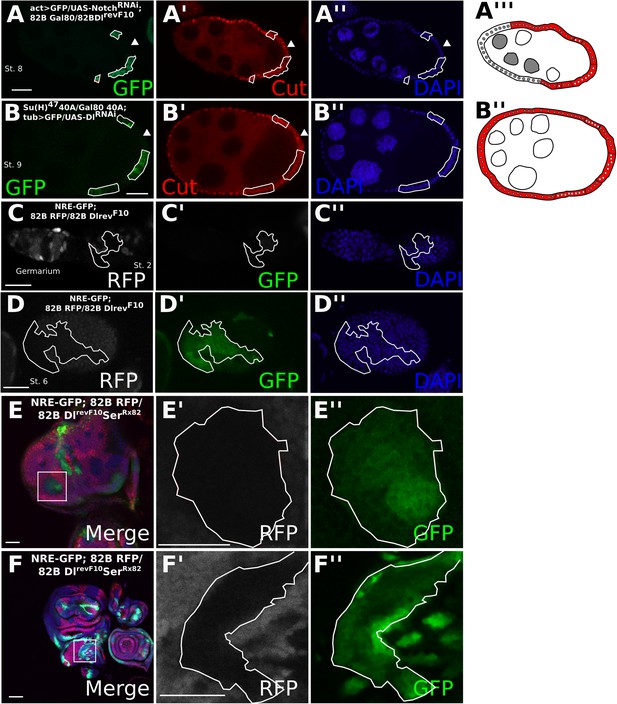
Cis-ligand represses ligand-independent Notch activity in the follicle cells and imaginal discs.
DlrevF10 mutant MARCM germline/follicle cell clones co-expressing NotchRNAi show prolonged Cut expression (A). Su(H)47 MARCM mutant germline/follicle cell clones co-expressing DlRNAi show failure to enter the endocycle (B). Germline clones are shown by late Cut expression in wild-type follicle cells (A, B, see arrowheads). See Figure 2—figure supplement 1A for control DlRNAi-induced germline follicle cell clones. Notch Responsive Element-green fluorescent protein (NRE-GFP) is upregulated beginning from stage 2 (C) and through later stages (D) in DlRevF10 germline and follicle cell clones. NRE-GFP is also upregulated cell-autonomously in DlRevF10SerRx82 mutant clones in eye (E) and wing (F) imaginal discs. Scale bars represent 20 μm.
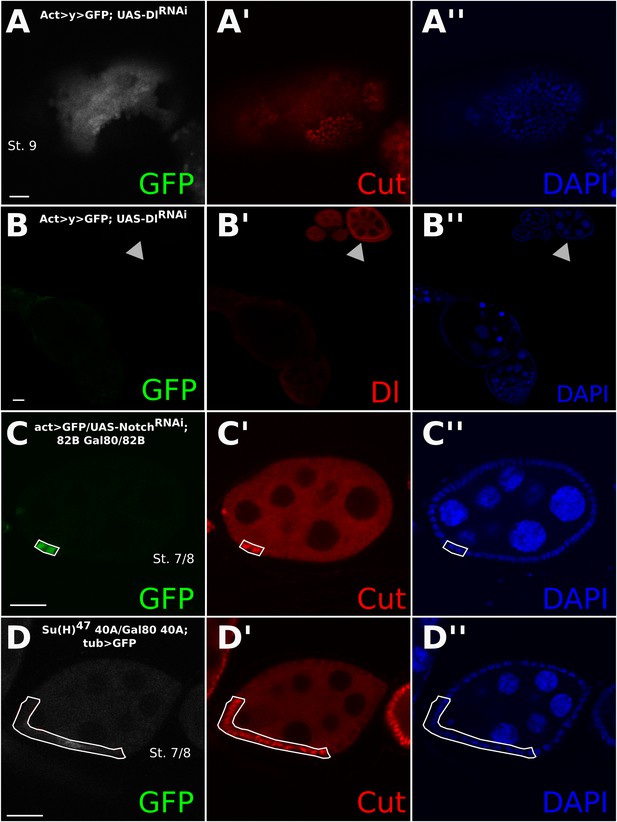
Control experiments relating to Figure 2.
The Dl-/Dl- phenotype can also be recapitulated using DlRNAi, which knocks down Dl in both the germline and soma using the FLP-out method (A and B). See the arrowhead in (B) for wild-type (WT) Dl staining. Again, germline clones are evidenced by aberrant Cut expression in WT follicle cells. MARCM-induced clones expressing only NotchRNAi show late Cut expression (C). Su(H)47 mutant clones created using the MARCM system also show late Cut staining and smaller nuclei (D). Scale bars represent 20 μm.
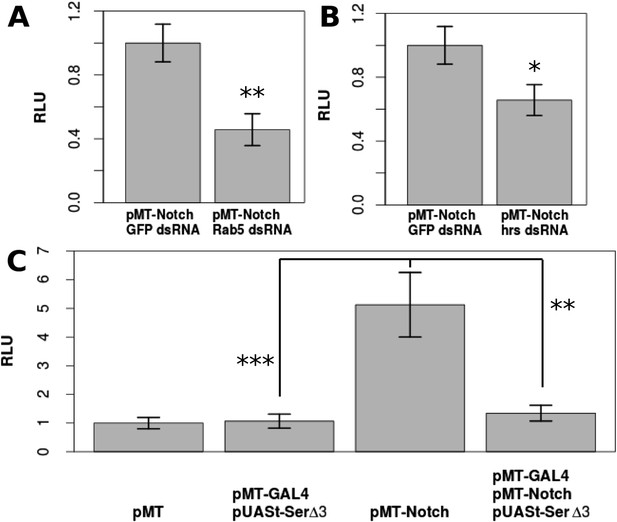
DSL-ligand-independent Notch activity in S2 cells is buffered by cis-ligand.
Trafficking is important for Notch activation in S2 cells, as treatment with Rab5 dsRNA (A) or hrs dsRNA (B) significantly decreases the amount of Notch activated in S2 cells as shown by Notch-responsive luciferase activity (NRE-firefly) in relative light units (RLU). Transfecting only pMT-NotchFL into S2 cells causes a 5.13-fold increase in Notch activation, which is almost entirely reduced (1.34-fold from the negative control) by co-transfection of pMT-GAL4 and pUASt-Serdel3 (C). Each experiment was carried out with two technical replicates and three biological replicates. Means of the technical replicates were used to carry out a paired t-test (n = 3) for each comparison. Error bars represent standard deviation (SD).
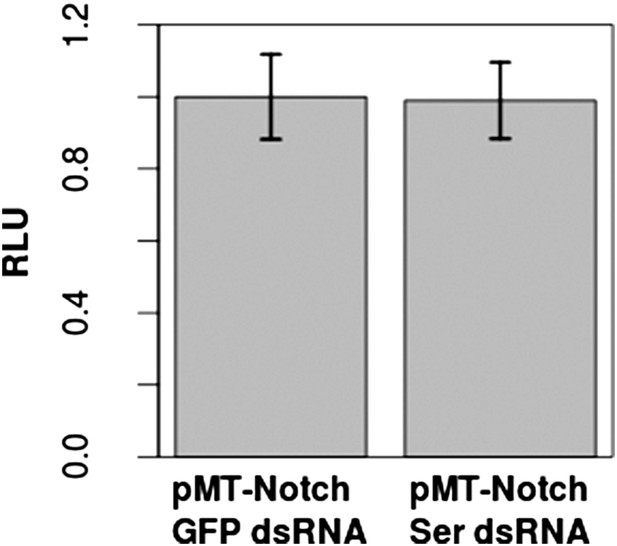
Addition of Ser dsRNA had no effect on the Notch activation in S2 cells in comparison with cells treated with control green fluorescent protein (GFP) dsRNA, indicating that the small amount of Ser expression is either not translated or does not significantly contribute to Notch activation upon transfection with pMT-NFL.
This validates our assumption that the Notch activation which occurs in S2 cells is by a DSL-ligand-independent mechanism. Dl was not tested, as studies have already shown a lack of Dl mRNA and protein in S2 cells (Fehon et al., 1990; Graveley et al., 2011). Again, experiments were carried out with two technical replicates and three biological replicates, with means of the technical replicates used for a paired t-test to assess significance. Error bars represent SD.
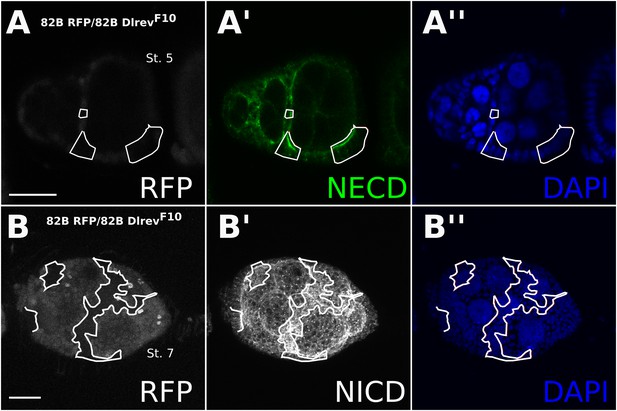
Notch accumulates in Dl-/Dl- clones.
Staining either Notch extracellular domain (A) or intracellular domain (B) showed increased Notch levels in DlrevF10 mutant germline/follicle cell clones. This could be seen as early as stage 2 where Notch protein seemed membrane localized (A), but by stage 5 it no longer localized to the membrane and appeared as a somewhat cloudy cytoplasmic accumulation (B). Scale bars represent 20 μm.
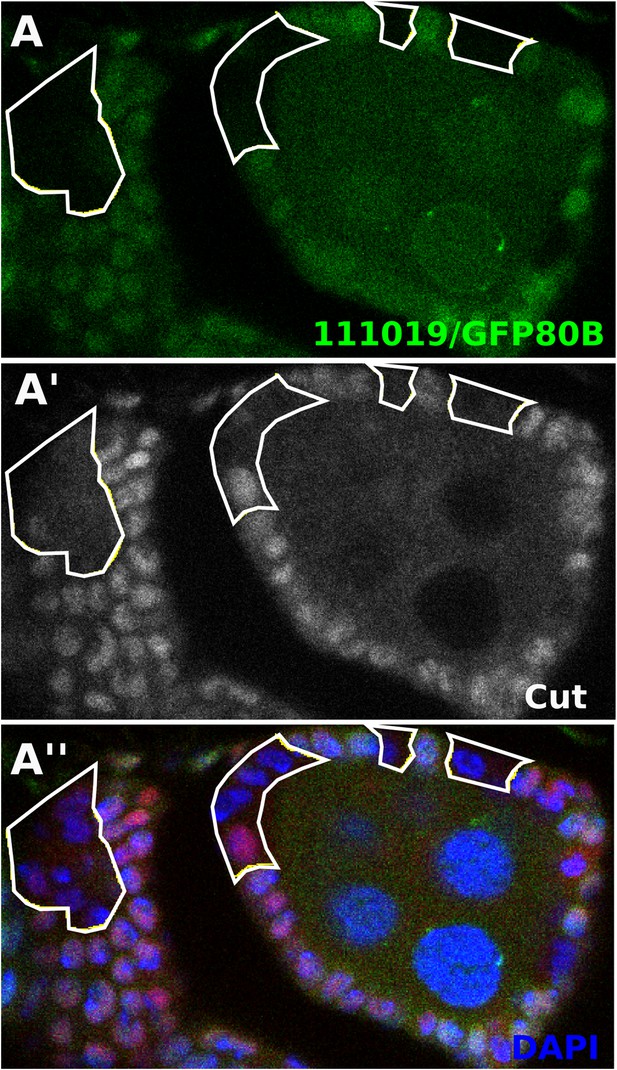
Follicle cells mutant for ESCRT component tsg101 show early Notch activity in the follicle cells (Vaccari et al., 2008).
tsg101111019 clones show early Cut downregulation.
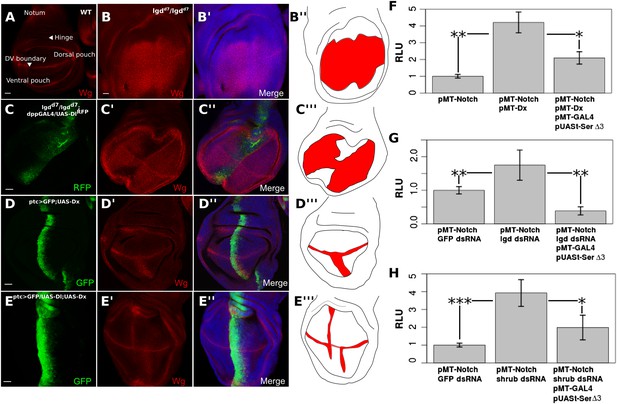
Notch ligand buffers against genetically induced DSL-independent activation.
Wing discs were stained with Wg antibody and illustrations are colored red where Wg is expressed (A–E). A wing disc with regions of interest is labeled and WT Wg staining shown (A). lgdd7/lgdd7 wing discs show ubiquitous Wg expression in the wing pouch as a result of DSL-ligand-independent Notch activity (B). Misexpression of UAS-Dl in lgdd7/lgdd7 discs causes a reduction in Wg staining along the anteroposterior boundary of the pouch (C). ptcGAL4 drives UAS-Dx causing ectopic Notch activity in the ventral wing pouch (D). Co-expression of Dx with Dl reduces Wg staining in the ptc domain (E), although, as in lgdd7/lgdd7 discs, the reduction is not complete towards the dorsoventral boundary. Cis-ligand also decreases Notch activation caused by genetic defects in S2 cells (F–H). Co-transfection with pMT-NFL and pMT-Dx caused a significant increase in Notch luciferase reporter expression, and adding Serdel3 significantly reduced this Dx-induced activation (F). Cells treated with lgd dsRNA (G) or ESCRT-III component, shrub, dsRNA (H) also caused significant increases in Notch reporter activity, either of which could be blocked by addition of Serdel3. For each of the S2 cell experiments, means were taken for technical duplicates and used for a paired t-test for three biological replicates. Error bars represent SD. Scale bars represent 20 μm.
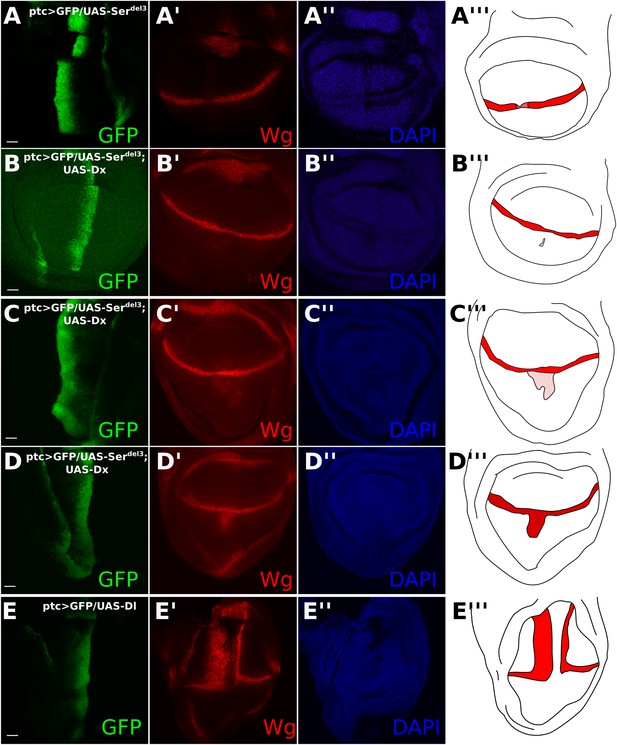
Co-expression of UAS-Dx and UAS-Serdel3 has a variable effect on DSL-independent Notch activation.
Wing discs were stained with Wg antibody (A–E). Illustrations show Wg staining in red, with lower intensities of Wg presence being shown in pink (A–E). ptcGAL4 driving green fluorescent protein (GFP) and UAS-Serdel3 along the anteroposterior (AP) boundary (A). This caused an incomplete reduction in Wg staining along the dorsoventral (DV) boundary. The slight increase in the red channel along the AP boundary is because the UAS-Serdel3 construct is tagged with tomato and bleeds into our ‘red’ secondary antibody confocal channel. For the rest a different channel was used. Coexpression of UAS-Serdel3 with UAS-Dx showed a variable effect on the Dx-induced aberrant Notch activity (B–D). Sometimes Dx-induced Notch activity was completely abolished (B), sometimes only partially reduced (C), and sometimes remained unchanged (D). All UAS-Serdel3/UAS-Dx discs are from the same round of antibody staining and taken with the same scale and settings on confocal microscopy. ptcGAL4 driving UAS-Dl caused a complete reduction of Wg at the DV boundary and elicited aberrant Wg expression on the boundary of the ptc domain. (E) Scale bars represent 20 μm.
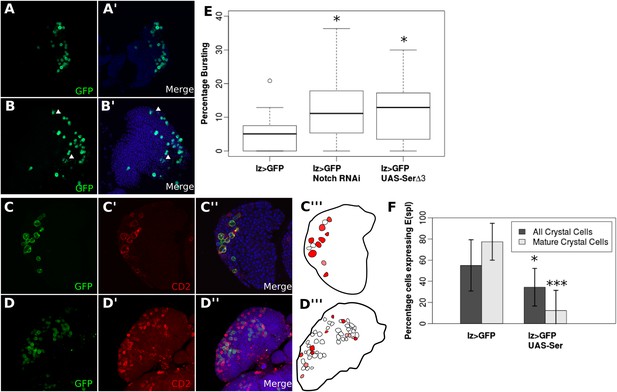
Endogenous DSL-independent Notch activity in crystal cells is reduced by cis-inhibition.
Lz-GAL4-driven green fluorescent protein (GFP) expression is an efficient marker of crystal cells which show a low incidence of bursting (A and E). Misexpressing UAS-Serdel3 increased the frequency of witnessing bursting crystal cells (see arrowheads in B) (B and E). Lymph glands were counted for each genotype (n = 14 for lz > GFP, n = 12 for lz > GFP; UAS-NotchRNAi, and n = 14 for lz > GFP; UAS-Serdel3). Welch's t-test was used to assess significance between wild-type (WT) lymph glands and each of the experimental groups (p = 0.043 and p = 0.029, respectively). To determine whether Serdel3-misexpression induced ‘bursting’ was caused by the cis-inhibitory effect of Ser on ligand-independent Notch activation, we used the Notch activity reporter E(spl):mβ-CD2. We focused our analysis on larger crystal cells, which enter the endocycle as part of their differentiation (Terriente-Felix et al., 2013), and therefore are the ones most probably undergoing ligand-independent Notch activation. For illustrations, all GFP-positive cells were outlined and were filled in with differing shades of red corresponding to Notch reporter staining intensity. E(spl)mβ:CD2 is expressed in 55% of crystal cells and 77.4% of mature crystal cells (C and F). Misexpression of UAS-SerWT significantly (p < 0.0001 for mature crystal cells, p = 0.0457 if all crystal cells were taken into account) reduced the fraction of crystal cells which show E(spl)mβ:CD2 expression, with 34.4% of all cells showing expression and 20.7% of mature cells showing CD2 expression (D and F). For this analysis, the total number of lz > GFP cells were counted, taking into account their size, lz > GFP intensity, and E(spl)CD2 intensity. Mature crystal cells were defined as cells that were both large and had intense lz > GFP. We then took the proportion of either all cells, or mature cells which had E(spl)CD2 staining for each lymph gland (n = 12 for lz > GFP, n = 13 for lz > GFP;UAS-Ser). Grubbs' outlier test was used, which removed one data point (p < 0.05) from the control, which had an unusually small number of crystal cells. Then Welch's t-test was used to assess significance between the mean proportions of crystal cells which showed Notch activity. All error bars represent SD. These observations indicate that increased ligand expression in crystal cells decreases cell survival by blocking Notch ligand-independent activation, and therefore the buffering role of cis-expressed ligand can be extended to endogenous cases of DSL-independent Notch activity. Scale bars represent 20 μm.
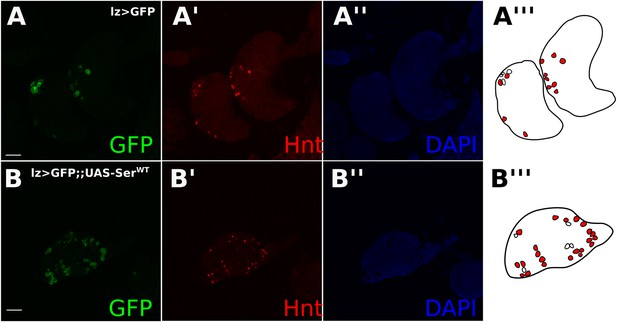
Reduced Notch reporter activity in crystal cells was not caused by indirect effects on early ligand-dependent Notch signaling in prohaemocytes.
Normal Hnt expression in crystal cells expressing green fluorescent protein (GFP) driven by lz-GAL4 (A) and in lz-GAL4 driving expression of UAS-SerWT (B). There was no noticeable effect on the proportion of cells expressing Hnt when UAS-SerWT was misexpressed. Illustrations show outlines of crystal cells with either no Hnt expression (white filling) or Hnt expression (red filling). Scale bars represent 20 μm.
Additional files
-
Supplementary file 1
Supplementary clonal data file. Excel worksheet containing clonal data from the egg chamber and the wing disc.
- https://doi.org/10.7554/eLife.04415.016





