High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation
Figures
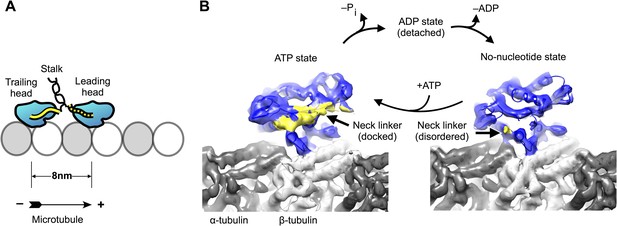
Kinesin-microtubule complex at 5–6 Å resolution.
(A) Schematic of a stepping kinesin dimer on a microtubule protofilament. (B) Cross-sections of the reconstructed co-complexes of no-nucleotide kinesin (left) and ADP•Al•Fx (right) with the microtubule, running parallel to a tubulin protofilament. Fourier Shell Correlation curves and other diagnostic information related to 3D refinement and reconstruction are found in Figure 1—figure supplement 1.
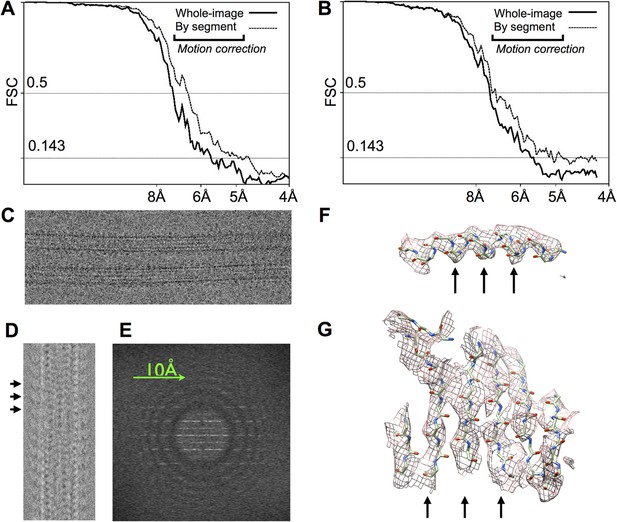
Statistics and diagnostic images related to image processing and 3D reconstruction of the kinesin-microtubule complex.
(A), (B) FSC curves for our 3D reconstructions of microtubules decorated by no-nucleotide and ADP•Al•Fx bound kinesin (respectively). These show signal nearly to the Nyquist frequency, dropping below the 0.143 threshold (1) in the 5–6 Å range. Bold lines correspond to reconstructions in which motion correction was only applied to the initial images. Thin lines reflect a gain of 0.5–1 Å in resolution that was achieved by dividing each box segment into three sub-averages of five video frames each, and then separately refining the position and Euler angles of the three sub-averages. (C) Representative image of the ADP•Al•Fx sample, after motion correction. (D) Post-processed diagnostic image for a 14-protofilament microtubule similar to that shown in (C), depicting the filament in a longitudinally compressed form. Each visible 8 nm repeat (marked with black arrows) corresponds to ∼8 repeats in the original filament image that have been averaged together, yielding an 8-fold shortened image with improved signal-to-noise ratio. Note that the contrast has been inverted in this image compared with (C). (E) Diffraction pattern obtained by straightening the microtubule imaged in (D) using in-plane orientation parameters obtained from the final cycle of reference alignment. Thon rings are visible to beyond 8 Å resolution in all directions. Possibly due to thick ice, Thon rings were rarely if ever observed to extend beyond ∼6 Å resolution, despite the combination of high-voltage instrument and direct electron detectors used in the current work. (F) Example of alpha-helical density from tubulin where helical pitch (arrows) can be resolved. (G) Example of beta-sheet density where individual strands are partially resolved (arrows).
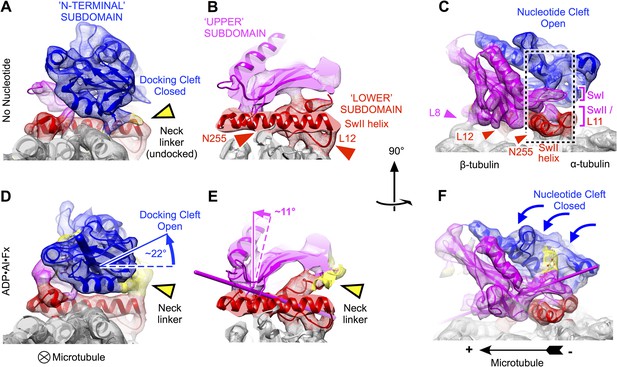
Clamshell-like closure of kinesin's nucleotide cleft triggered by ATP binding on microtubules, as revealed by cryo-EM density maps of no-nucleotide kinesin (top panels) and the ADP•Al•Fx state (bottom panels).
Nucleotide cleft closure is coupled, via rotation of the N-terminal subdomain, to opening of a ‘docking cleft’ on the opposite side of the motor domain and accompanying docking of the neck linker (A), (B), (D), (E) two different slices of density perpendicular to the microtubule axis, detailing the N-terminal and upper domains (respectively). Microtubule contacts are denoted by colored arrowheads. (C), (F) Side view of motor domain facing the nucleotide active site. Thin colored cylinders in panels D–F depict rotational axes that describe re-orientations of the N-terminal domain (blue) and upper domain (magenta) compared with the no-nucleotide state. Atomic models superposed on these maps in this and the following figures were derived from our cryo-EM data by molecular dynamics flexible fitting simulations as described in the text (see Videos 1–3 and Figure 2—figure supplements 1–3).
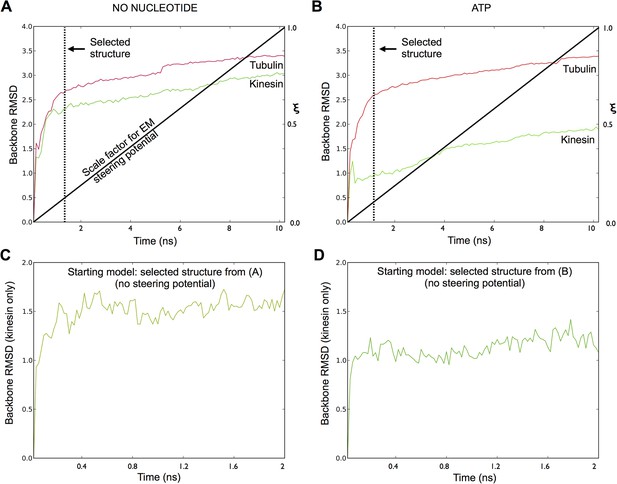
Assessing the convergence of the MDFF simulations.
(A–B), Backbone RMSD values as a function of time, measured separately for tubulin and kinesin against the starting model, for MDFF simulations of the no-nucleotide and ATP states of kinesin (respectively). The bold black line indicates the corresponding value of the EM potential scaling factor (ξ), which follows a linear ramp function (See ‘Materials and methods’). Time points selected to represent the fitted complex (with minimal over-refinement artifacts) are indicated by dashed lines. (C–D), Backbone RMSD values in follow-up simulations starting with the selected structures in (A) and (B), respectively, where the cryo-EM potential was turned off but the coordinates of tubulin were restrained in the ‘straight’ conformation (see ‘Materials and methods’).
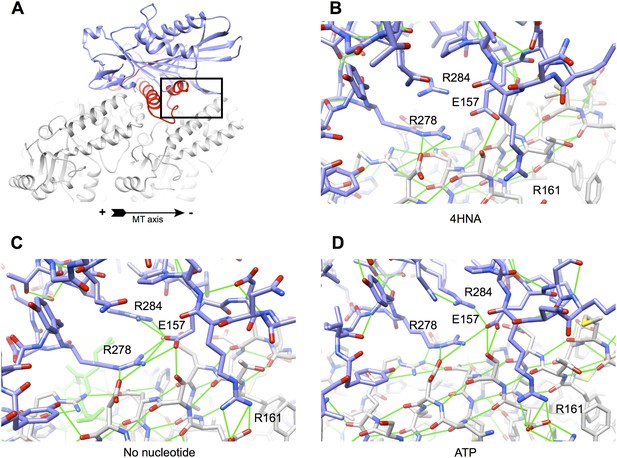
Improved interactions at the kinesin-tubulin interface following initial equilibration of the 4HNA starting model (see ‘Materials and methods’).
(A) Overview of the kinesin-microtubule complex, highlighting the region selected for the close-ups in panels (B)–(D). Four charged residues from kinesin's microtubule binding regions are labeled. The hydrogen-bonding geometry for R278 (loop L12) is extremely poor and R161 has no interaction partners in the 4HNA starting model (B), likely due to resolution limitations and anisotropy in the X-ray data. In both the no-nucleotide and ATP simulations (C and D, respectively), these residues stably interact with complementary charge partners in tubulin and/or adjacent residues of kinesin.
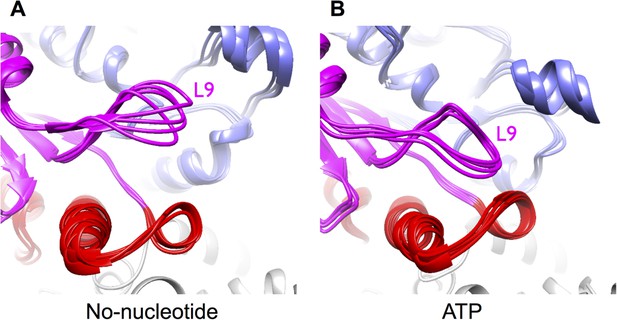
Mobility of loop L9 (corresponding to the switch I loop) increases in simulations of unrestrained, no-nucleotide kinesin bound to microtubules, compared with simulations of the ATP state.
These loop motions account for differences in cryo-EM density features for this loop seen in Figure 2C,F. These simulations were restarted from the endpoint of the corresponding MDFF simulations, but with the cryo-EM energy term turned off (see ‘Materials and methods’). The kinesin structure in these simulations exhibits substantially larger conformational fluctuations when compared to the MDFF simulations (not shown), indicating that addition of the cryo-EM energy term substantially restricts breathing movements that ordinarily occur at the simulated temperature (300 K). (A) Four representative conformations in the trajectory of unrestrained no-nucleotide kinesin, revealing that loop L9 can flap away from the nucleotide pocket when the gamma phosphate is absent. (B) Four representative conformations from the ATP-bound kinesin trajectory, reflecting the fact that L9 is stably engaged with the gamma-phosphate and is unable to fluctuate greatly.
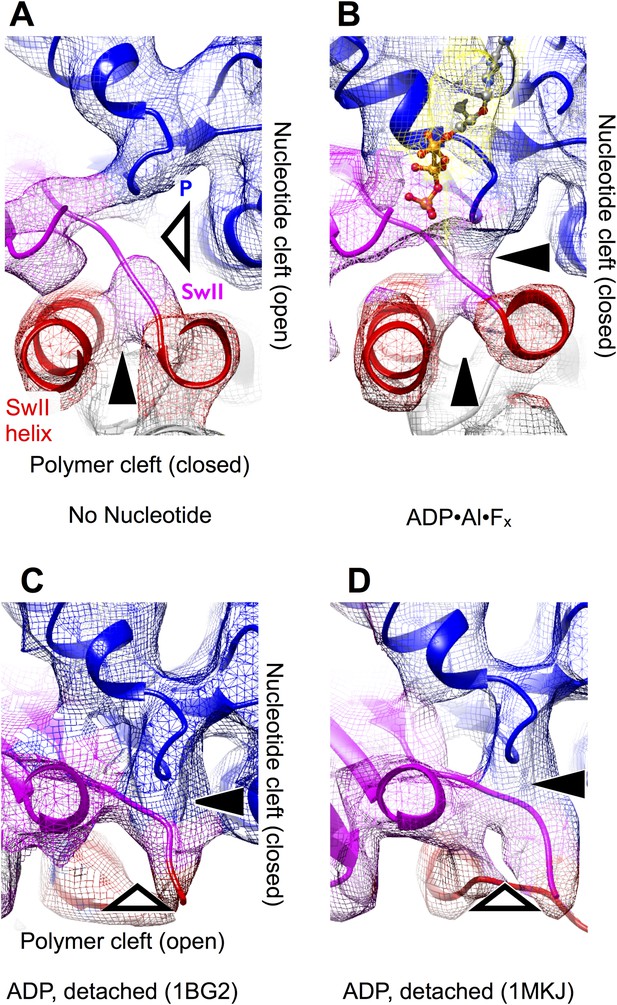
The effect of nucleotide state and microtubule attachment on kinesin’s ‘nucleotide’ and ‘polymer’ clefts.
Shown is an enlarged, cutaway view of the active site (rectangular region indicated in Figure 2C,F). Density features that define the nucleotide cleft, corresponding to the switch II loop and the P-loop, are indicated by ‘SwII’ and ‘P’ (respectively) in panel A. Hollow and solid wedges denote open and closed states (respectively) of the nucleotide and polymer clefts. Panels A–B show isosurface renderings our cryo-EM maps, while panels C–D show synthetic density maps generated from X-ray structures of our identical kinesin construct in its ADP-bound state, filtered to a similar resolution (6 Å) as the experimental maps in panels A–B. Figure 3—figure supplemental 1 shows the corresponding features for synthetic 6 Å-resolution maps generated from our atomic models, as well as for two additional ADP-bound crystal structures of kinesin.
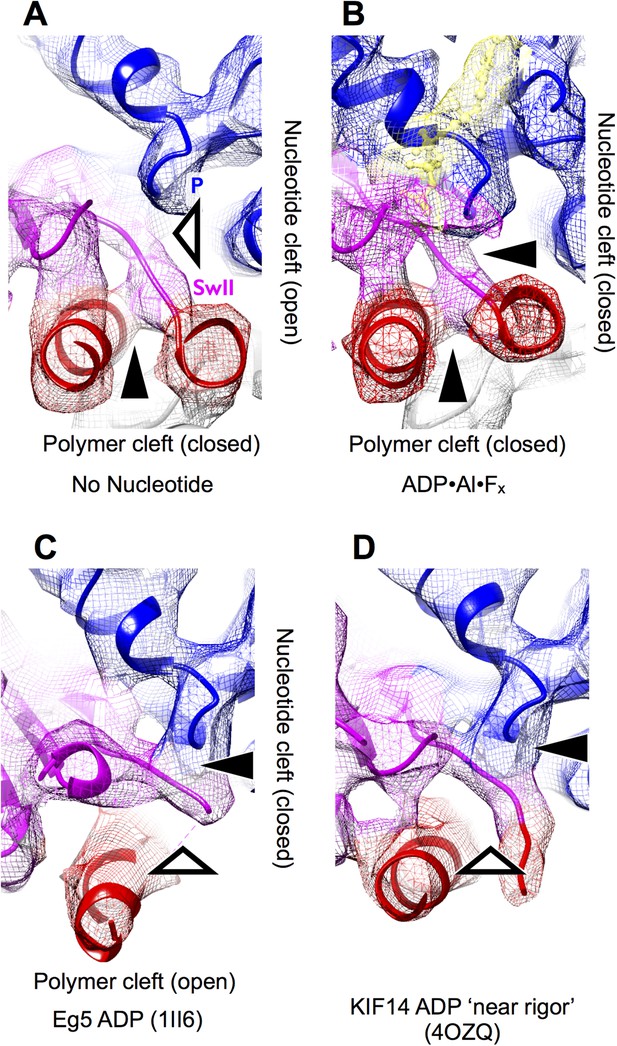
Visualizing nucleotide and polymer clefts in synthetically rendered maps of kinesin at 6 Å resolution.
The view is the same as in Figure 3. Panels (A–B) were generated from the final atomic models for these maps generated by our MDFF simulation protocol, while panels (C–D) were rendered from the listed PDB coordinates.
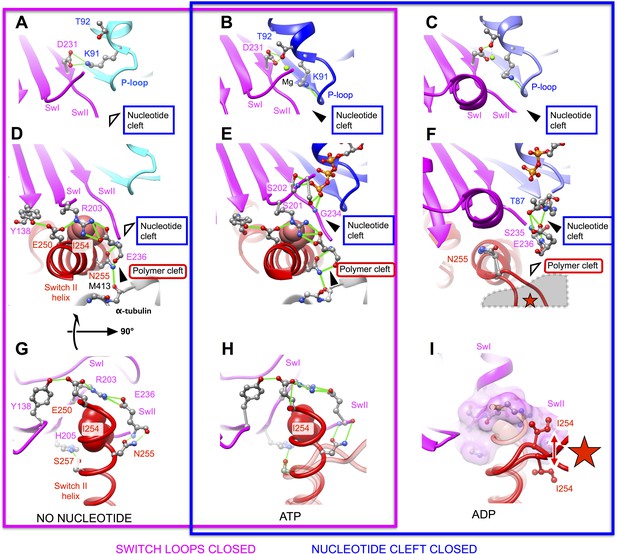
Atomic models capturing open and closed states of kinesin's nucleotide and polymer clefts for principle structural intermediates in the kinesin cycle.
No-nucleotide and ATP-bound models of the kinesin-microtubule complex (this work) are depicted in panels A,D,G and B,E,H (respectively). A pair of X-ray crystal structures of the ADP-bound kinesin K349 construct (PDB ID: 1BG2, 1MKJ, which have undocked and docked neck linkers, respectively) are superposed in C, F and I. Panels G–I show rotated views of the structures, presenting the view from the microtubule interior looking outward at the kinesin interface. Universally conserved residues involved in the depicted structural transitions are rendered as ball and stick figures, and hydrogen bonds are depicted as solid green lines. Hollow and solid wedges denote open and closed states (respectively) of the nucleotide cleft and the polymer cleft, while the star in panels F and I indicates the site of predicted steric clashes between the switch II helix and alpha tubulin. For comparison, views of the active sites of the F1-ATPase and myosin motors in no-nucleotide and ATP analog-bound states, corresponding to panels A and B, are shown in Figure 4—figure supplement 1.
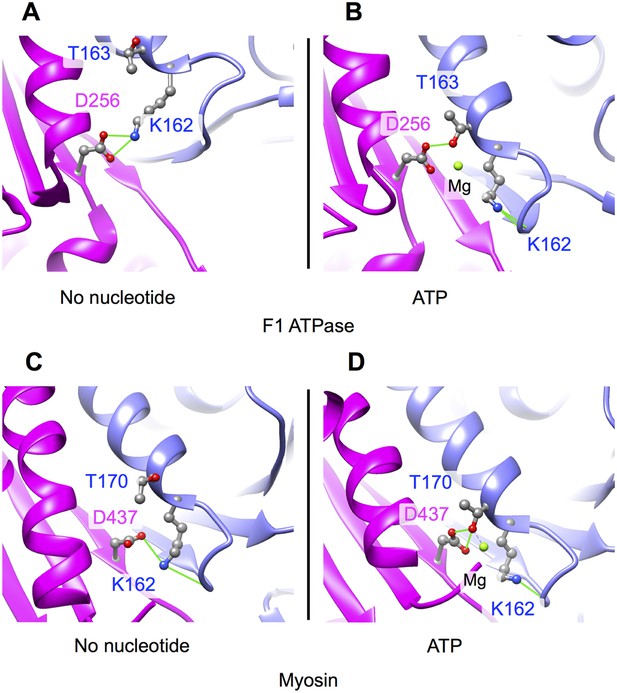
Nucleotide cleft closure induced by ATP analogs in the myosin and F1-ATPase motor proteins (compare with Figure 4A–C).
Changes in the hydrogen-bonding pattern between P-loop and switch II residues between in these motors closely match the changes predicted by our molecular dynamics models of kinesin's no-nucleotide and ATP states. Both of these other motors also undergo substantial distortions in the supporting beta-sheet conformation that accompany the P-loop movement. (A–B) Active site of the subunit of bovine mitochondrial F1-ATPase in no-nucleotide and ADP•Al•Fx states, respectively, from a crystal structure of the inhibited (αβ)3-γ co-complex (PDB ID: 1H8E) c-d. Active site of the myosin V motor domain from X-ray structures solved in the absence and presence of ADP•Al•Bex (PDB ID's 1W8J and 1W7J, respectively). Viewing orientations of the structure pairs in top and bottom panels are generated by aligning the backbone atoms in the beta-strand residues N-terminal to the switch II aspartic acid (D256 and D437, respectively).
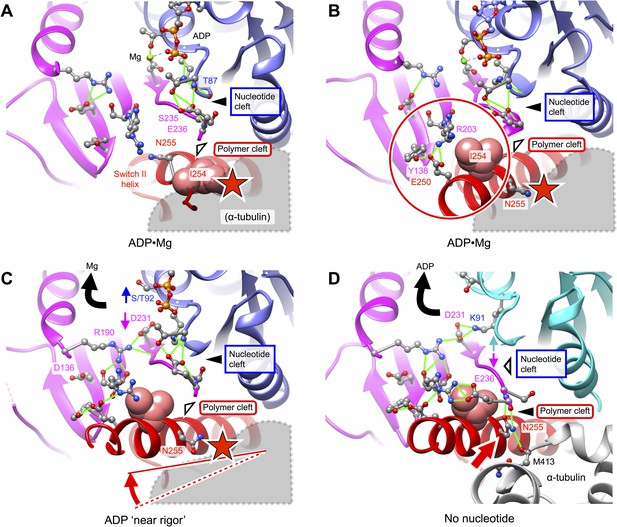
Putative intermediate states that lead to opening of kinesin’s nucleotide cleft, accompanied by ADP release, upon microtubule attachment.
(A) X-ray structure of Mg•ADP-bound K349 kinesin (PDB ID 1BG2). (B) X-ray structure of Mg•ADP-bound KIF1A kinesin (PDB ID: 1I5S). (C) X-ray structure of ‘near rigor’, ADP-bound KIF14 kinesin (PDB ID: 4OZQ) (D) No-nucleotide kinesin-microtubule complex (this work). Structures in panels A–C are aligned with the no-nucleotide K349 model in panel D by strand b7 of the central beta sheet, which connects to the switch II loop; this alignment approximately maintains the orientation of the upper subdomain, thus preserving a contact between L8 within this subdomain and alpha tubulin (see Figure 2). In panels A–C, predicted steric clashes between alpha tubulin and residues corresponding to I254 and/or N255 (K349 numbering) are marked with a red star.
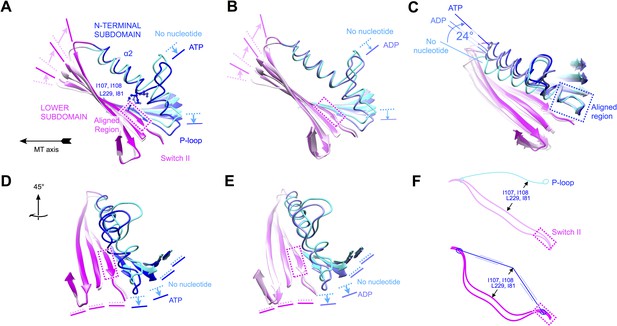
Evidence that nucleotide binding introduces internal strain in kinesin's central beta sheet and helix alpha 2, mediated by interactions between the P-loop and the switch II loop.
Similar comparisons of additional structural states of kinesin are shown in Figure 6—figure supplement 1 and Videos 4–8. (A) Flexing of the beta sheet coupled with motion of alpha 2 in comparisons of no-nucleotide and ATP-bound conformations of microtubule-attached kinesin (this work). Structures are aligned by central region of the beta strand adjoining the switch II loop (residues 226–231). (B) Comparison of no-nucleotide and ADP-bound (1MKJ) structures of kinesin, aligned as in panel A. (C) Comparison of no-nucleotide (current work), ADP-bound (1BG2 and 1MKJ), and ATP-bound (current work) states of the K349 kinesin construct aligned by their P-loops, revealing nucleotide-dependent flexing of helix alpha 2. (D), (E) Rotated views of panel A and B, respectively, highlighting distortion in the beta sheet proximal to the P-loop. (F) Cartoon depicting the inferred energy storage mechanism, by analogy to stringing an archery bow. The strain appears to be magnified by a cluster of conserved side chains (I107, I108, L229, I81; see panel A) that separate alpha 2 and the beta sheet midway along the interface between these elements.
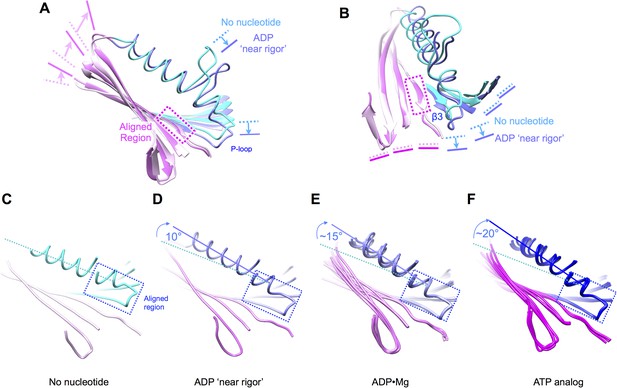
Relationship between nucleotide state and strain in the polypeptide backbone for a diverse array of kinesin X-ray structures.
(A), (B) Comparison between our no-nucleotide K349 structure and a ‘rigor-like’ (ADP-bound) conformation of KIF14 (PDB ID: 4OZQ). Compare with Figure 6A,B,D,E. (C–F) Comparison of the orientation of helix α2 relative to the P-loop, for various crystalized states of kinesin. All alignments in these panels are performed relative to the backbone atoms of residues 85–92 (the P-loop) in our no-nucleotide structure (shown in (C)). The 4OZQ structure is shown in (D). In (E), five representative structures of ADP•Mg-complexed kinesin are superposed (PDB ID's: 1BG2, 1MKJ,1I5S, 1II6, 1F9V); in (F), our ATP state model is depicted along with the three currently available X-ray structures of kinesin in which the switch loops are fully closed (all co-bound with ATP analogs) (PDB ID's: 3HQD, 3ZFC, 4HNA).
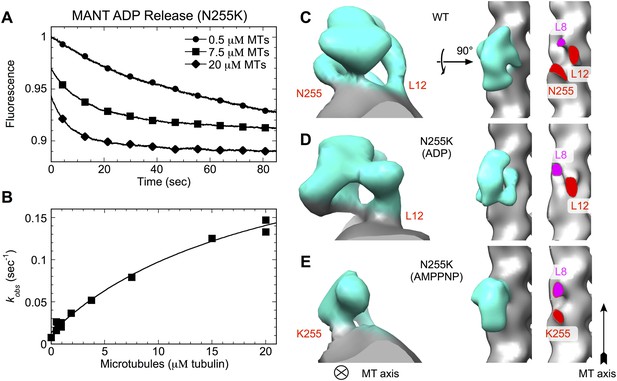
N255K uncoupling mutation compromises the microtubule interface, with accompanying orientational disorder of the motor domain.
(A), (B) Microtubule-stimulated ADP release kinetics measurements for the N255K mutant of our K349 construct. Shown in (A) are individual time traces, while (B) plots the fitted rate constants as a function of microtubule concentration. The extrapolated maximum rate for microtubule-stimulated ADP release was kmax = 0.26 ± 0.04 s−1, with an apparent microtubule dissociation constant of K0.5 = 21.3 ± 6.3 μΜ. (C–E) Cryo-EM reconstructions of microtubules decorated with wild-type K349 (Sindelar and Downing, 2010) and the N255K mutant. For the wild-type structure in (C), the ADP state was selected for viewing purposes but all nucleotide states have similar features at this resolution. Maps from reconstructions of 14-protofilament microtubules are shown; features in the corresponding 13-protofilament reconstructions were very similar (results not shown). All maps are low-pass filtered to 18 Å resolution, corresponding the estimated resolution of the N255K reconstructions. Supporting information describing cryo-EM analysis and 3D reconstruction of the N255K samples are shown in Figure 7—figure supplement 1.
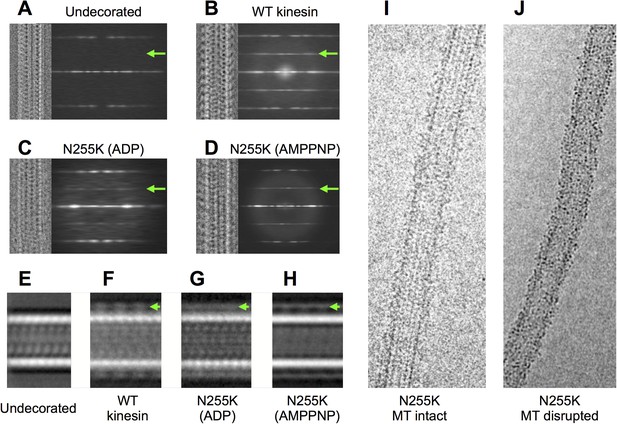
Analysis of cryo-EM images of N255K kinesin complexed with microtubules (see Figure 7).
(A–D) Compressed alignments (similar to Figure 1—figure supplement 1D) of representative single microtubules under various decoration conditions. A summation of the power spectra for every selected microtubule of the corresponding condition is shown to the right of each compressed microtubule image. All micrographs were collected under similar imaging conditions (magnification, voltage, defocus, etc), using the same equipment (FEI TF20 electron microscope equipped with a Gatan US4000 CCD camera). For comparison, analysis of an undecorated microtubule data set is shown in (A). A green arrow indicates the location of the 80 Å layer line in these power spectra, present only in the cases where there is motor decoration. Compared with the wild-type K349 construct (panel (B)), the N255K mutant (panels (C), (D)) binds to microtubules but the 80 Å layer line due to motor binding is significantly weaker. The weaker 80 Å diffraction signal is indicative of poor ordering and/or occupancy of the attached motor heads. (E–H), images formed by summing aligned box segments corresponding to the samples in (A–D). Visible density for kinesin in these summations (green arrows) indicates that the N255K construct decorates microtubules with similar efficiency as the wild-type construct, despite weaker 80 Å diffraction by the mutant. This observation was supported by the ability of the N255K mutant to co-sediment with microtubules even in the ADP condition (see ‘Materials and methods’). Therefore, weak 80 Å diffraction in (C) and (D) must originate from heterogeneity in conformation of the bound motor heads rather than low occupancy, consistent with the reconstructions shown in Figure 4 of the main text. (I) Image of a microtubule decorated by the N255K construct (ADP condition). While obviously decorated, the microtubule has a shaggy appearance uncharacteristic of decoration by wild-type kinesin. (J) Example of a morphologically disrupted microtubule, frequently seen in cryo-EM specimens of microtubules decorated by the N255K construct. The disrupted microtubules were excluded from data sets used for image processing and 3D reconstruction.
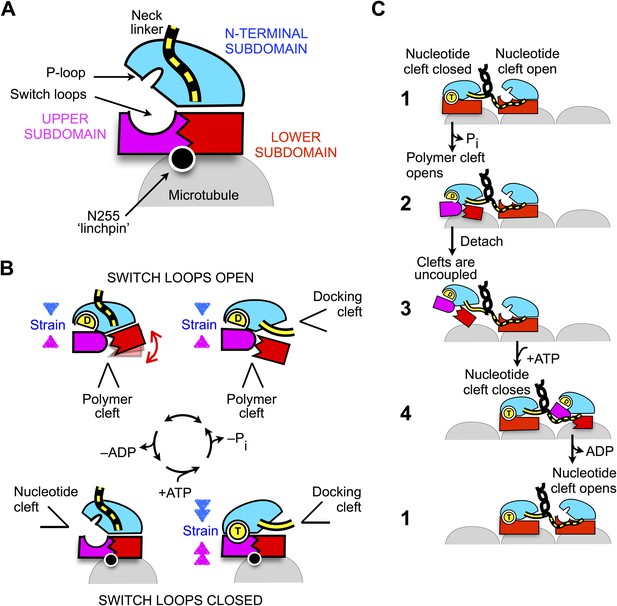
Schematic of proposed mechanisms of monomeric and dimeric conventional kinesin.
(A) Abstracted cartoon that depicts kinesin's three principal subdomains and other key structural elements, including the ‘linchpin’ residue N255 (circle) and the neck linker (disordered state depicted as dashed yellow lines). (B) Schematic reaction depicting four principal structural intermediates in the ATPase cycle of monomeric kinesin. States of the nucleotide, polymer, and docking clefts are indicated. (C) Alternating cleft model for processive stepping by dimeric kinesin.
Videos
Animation depicting the conformational transition observed in our MDFF simulation of the ATP bound kinesin–microtubule complex.
A ribbon diagram of the molecular structure is superimposed on a semitransparent isosurface of the EM density map, filtered to 6 Å resolution. The video runs from the beginning of the MDFF simulation up to t = 1.2 ns (corresponding to the selected final model). The second half of the video plays the same transition backward. Coordinates were extracted at 50 ps intervals from the simulation trajectory. To aid in viewing, the depicted coordinate trajectory was smoothed using a 5-frame sliding window, using the ‘smoothmd.py’ script available from the UCSF Chimera web site (http://plato.cgl.ucsf.edu/trac/chimera/attachment/wiki/Scripts/smoothMD.py).
Animation depicting our MDFF simulation for no-nucleotide kinesin, up to t = 1.4 ns.
https://doi.org/10.7554/eLife.04686.012Alternative depiction of the transition presented in Video 2, detailing the side chains and hydrogen bonds that compose the closed switch loop network (Y138, R203, E236, E250, N255), as well as residues P-loop residues K91, T92, and their interacting partner D231 from the switch II loop (compare with Figure 3).
Hydrogen bonds formed at the beginning and endpoints of the simulation are depicted with green lines. Note that in this video the position and orientation of the outer surface of tubulin (helices H11-H12) is held fixed. In order to facilitate looping presentations of the video, the second half of the video presents the same frames of the MDFF trajectory but in reverse order.
Comparison of beta-sheet twist in our no-nucleotide model (cyan) and five representative X-ray structures of ADP-bound kinesin (pale magenta/pale blue).
Structures in this and all subsequent videos are aligned by residues 226–231 of our kinesin model, as in Figure 6A. The video cycles sequentially through the ADP structures in order of increasing twist: frame 1, ADP-bound K349 (PDB ID:1MKJ); frame 2, ADP-bound KIF1A (PDB ID:1I5S); frame 3, ADP-bound K349 (1BG2); frame 4, ADP-bound KAR3 (PDB ID:1F9T); frame 5, ADP-bound Eg5 (PDB ID:1II6).
Similar to Video 4, but substituting our no-nucleotide model with the X-ray structure of ‘rigor-like’ kinesin, in green (PDB ID: 4OZQ).
https://doi.org/10.7554/eLife.04686.018Morphing animation illustrating minimal beta-sheet distortion in a comparison of ADP-bound structures of KIF1A (PDB ID:1I5S) and ‘rigor-like’ KIF14 (lacking magnesium; PDB ID:4OZQ).
The starting structure is that of KIF14. The hydrogen bond network between residues T87 and E236 (K349 numbering), which defines a closed state of the nucleotide cleft, is depicted and the involved side chains rendered as stick figures.
Comparison of backbone distortions in the central beta sheet and α2 for our no-nucleotide and ATP-bound models of K349 kinesin, illustrated by a morphing animation.
Compare with Figure 6A,D.
Similar to Video 7, but substituting the X-ray structure of ADP-bound kinesin (PDB ID: 1MKJ) for the ATP-bound model.
Compare with Figure 6B,E.





