Identification of PLXDC1 and PLXDC2 as the transmembrane receptors for the multifunctional factor PEDF
Figures
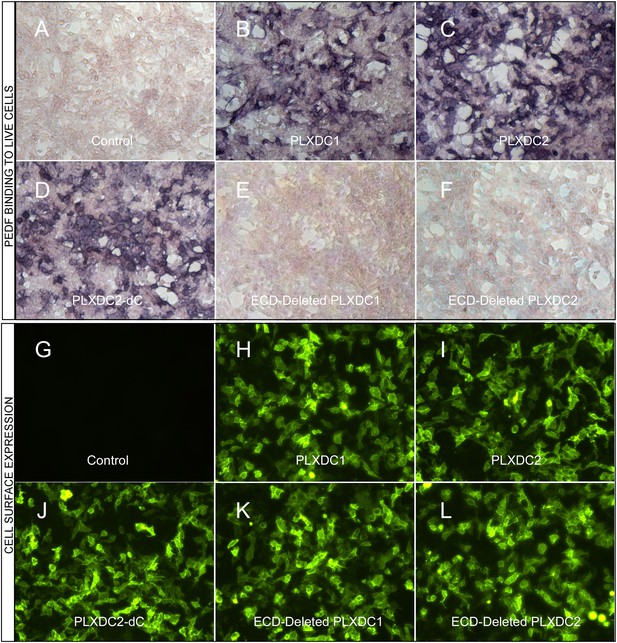
The binding of PEDF to PLXDC1 and PLXDC2 on cell surface.
Upper panel: Binding of biotinylated PEDF to control HEK293 cells (A) or HEK293 cells transfected with PLXDC1 (B), PLXDC2 (C), intracellular domain deleted PLXDC2 (D, PLXDC2-dC), extracellular domain (ECD) deleted PLXDC1 (E), or ECD deleted PLXDC2 (F). Binding was detected by stretavidin-alkaline phosphatase, shown as deep purple color. Lower panel: Live cell staining of an epitope tag of control HEK293 cells (G) or HEK293 cells transfected with PLXDC1 (H), PLXDC2 (I), intracellular domain deleted PLXDC2 (J, PLXDC2-dC), ECD-deleted PLXDC1 (K), or ECD deleted PLXDC2 (L). All constructs have the epitope tag engineered after the secretion signal at the N-terminus.
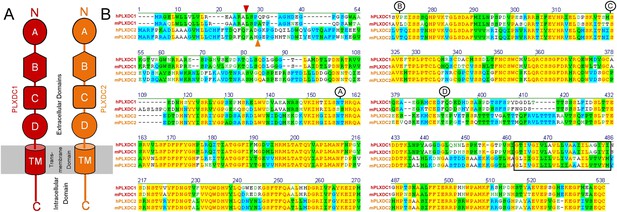
PLXDC1 and PLXDC2 schematic diagrams and alignment showing the definitions of domains.
(A) Schematic diagrams of domains in PLXDC1 (red) and PLXDC2 (orange). (B) Alignment of human (h) and mouse (m) PLXDC1 and PLXDC2. Junctions of domains are indicated. Identical regions are highlighted in yellow. The junction between the secretion signal and the rest of the protein is indicated by a red arrowhead (PLXDC1) or an orange arrow head (PLXDC2). The junction between domain A and B is indicated by the letter A in a circle. The junction between domain B and C is indicated by the letter B in a circle. The junction between domain C and D is indicated by the letter C in a circle. The end of domain D is indicated by the letter D in a circle. Domain B contains a region that is homologous to nidogen. Domain D includes the plexin homology domain. Putative location of the transmembrane domain is indicated by a black square.
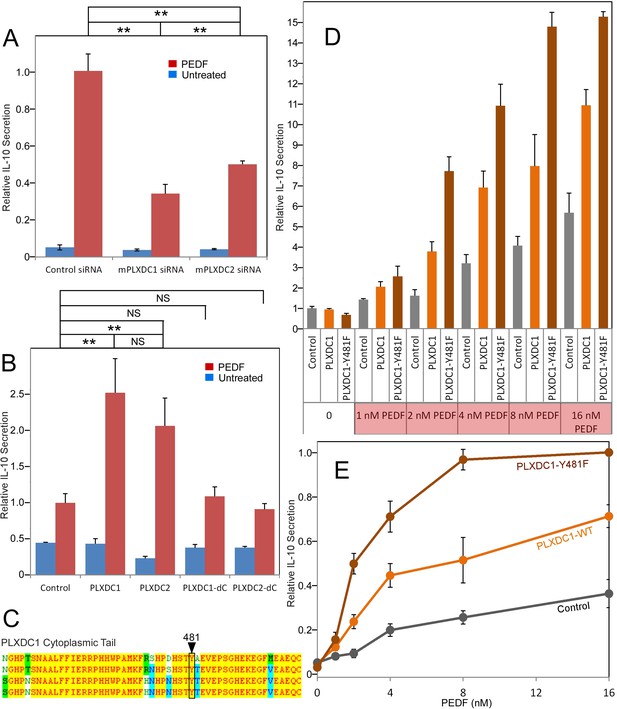
The roles of PLXDC1 and PLXDC2 in PEDF-induced IL-10 secretion by macrophage cell RAW267.4.
(A) siRNA-mediated knockdown of PLXDC1 or PLXDC2 substantially suppresses PEDF-stimulated IL-10 secretion. Activity of control transfected cells with PEDF treatment is defined as 1. ** = p < 0.01. (B) Transfection of either PLXDC1 or PLXDC2 cDNA enhances RAW cell's PEDF-stimulated IL-10 secretion, while PLXDC1 or PLXDC2 lacking the cytoplasmic domain (PLXDC1-dC or PLXDC2-dC) do not show significantly different secretion from control EGFP transfection. Activity of control cells with PEDF treatment is defined as 1. ** = p < 0.01; NS = not significant. (C) Alignment of human, bovine, mouse, and rat PLXDC1 cytoplasmic tail and the location of the putative phosphorylated residue (residue number according to human PLXDC1). (D) Comparing PEDF-induced IL-10 secretion by RAW267.4 transfected with PLXDC1 and PLXDC1-Y481F. Mutation Y481F on the cytoplasmic tail of PLXDC1 greatly enhances its response to PEDF. Activity of control transfected cells without PEDF treatment is defined as 1. (E) PEDF concentration-dependent stimulation of IL-10 secretion from control, PLXDC1, and PLXDC1-Y481F transfected cells (from D). Activity of PLXDC1-Y481F cells at 16 nM PEDF is defined as 1.
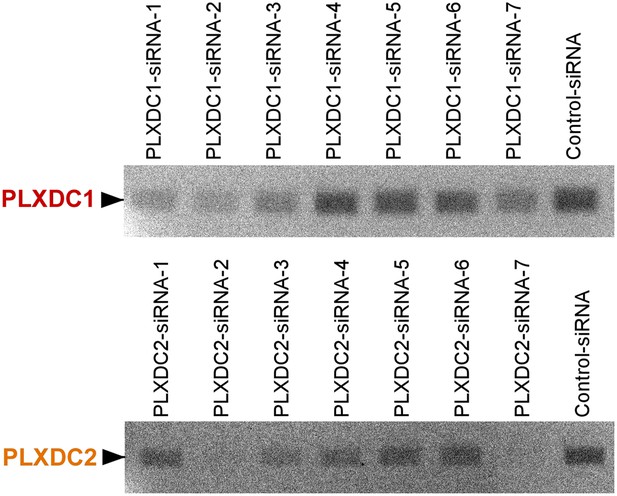
Unbiased screening for effective siRNAs that knock down PLXDC1 or PLXDC2 expression in macrophage cell RAW267.4.
The definition and source of each siRNA is described in ‘Materials and methods’.
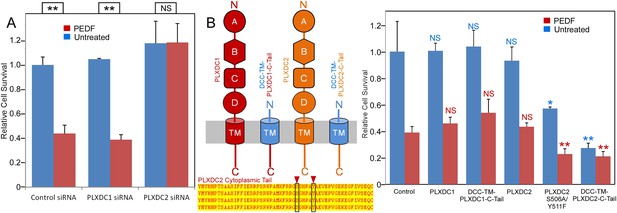
The PLXDC2 dependence of PEDF's effect on endothelial cell SVEC4-10.
(A) PEDF promoted-cell death of SVEC4-10 cells is suppressed by siRNA-mediated knockdown of PLXDC2, but not PLXDC1. Survival of control siRNA tranfected cells without PEDF treatment is defined as 1. Statistical significance is shown on the top. ** = p < 0.01, and NS = not significant. (B) Left panel: Schematic diagrams of full length receptors and the fusion proteins for the receptor cytoplasmic tails. The cytoplasmic tail of the receptor is fused to the TM domain of DCC, which is fused to the secretion signal of alkaline phosphase at the N-terminus. Alignment of human, mouse, rat and bovine PLXDC2 cytoplasmic tails shows complete conservation (bottom). Locations of potential phosphorylation sites are indicated. Right panel: Expression of cytoplasmic tail of PLXDC2, but not the cytoplasmic tail of PLXDC1 promotes SVEC4-10 cell death. PLXDC2 double mutant S506A/Y511F has greater activity. Survival of control EGFP transfected cells without PEDF treatment is defined as 1. Statistical significance of the comparison of cells without PEDF treatment (with the control cells without PEDF treatment) is shown in blue. Statistical significance of the comparison of cells with PEDF treatment (with the control cells with PEDF treatment) is shown in red. * = p < 0.05, ** = p < 0.01, and NS = not significant.
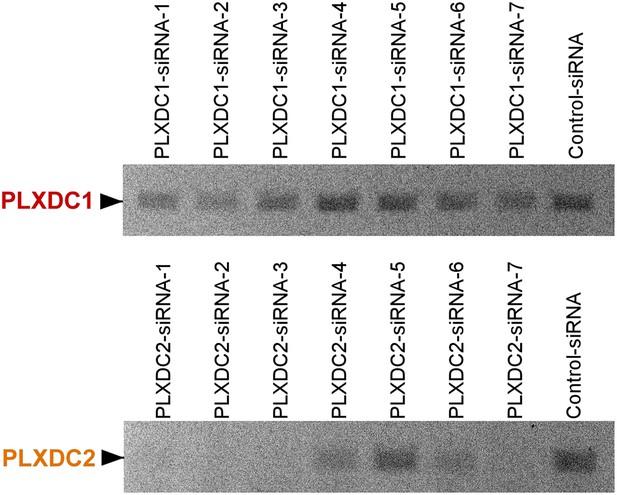
Unbiased screening for effective siRNAs that knock down PLXDC1 or PLXDC2 expression in endothelial cell SVEC4-10.
The definition and source of each siRNA is described in ‘Materials and methods’.
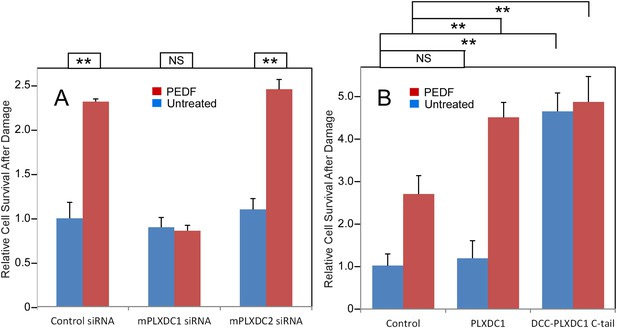
PEDF's neurotrophic effect on 661W cells depends on PLXDC1.
(A) PEDF treatment protects 661W cells against hydrogen peroxide-mediated oxidative damage. siRNA-mediated knockdown of PLXDC1, but not PLXDC2 abolishes the protective effect of PEDF. The survival of control siRNA transfected cell without treatment is defined as 1. (B) Transfection of PLXDC1 or the cytoplasmic domain of PLXDC1 fused to DCC's TM domain enhances protection of 661W cells against damage caused by hydrogen peroxide. The effect of DCC-PLXDC1 C-tail is independent of PEDF. Cell survival of transfected control is defined as 1. Statistical significance is shown on the top. ** = p < 0.01; NS = not significant.
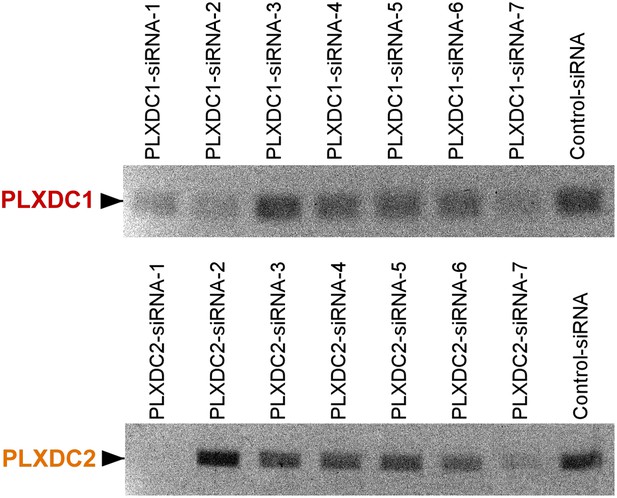
Unbiased screening for effective siRNAs that knock down PLXDC1 or PLXDC2 expression in neuronal cell 661W.
The definition and source of each siRNA is described in ‘Materials and methods’.
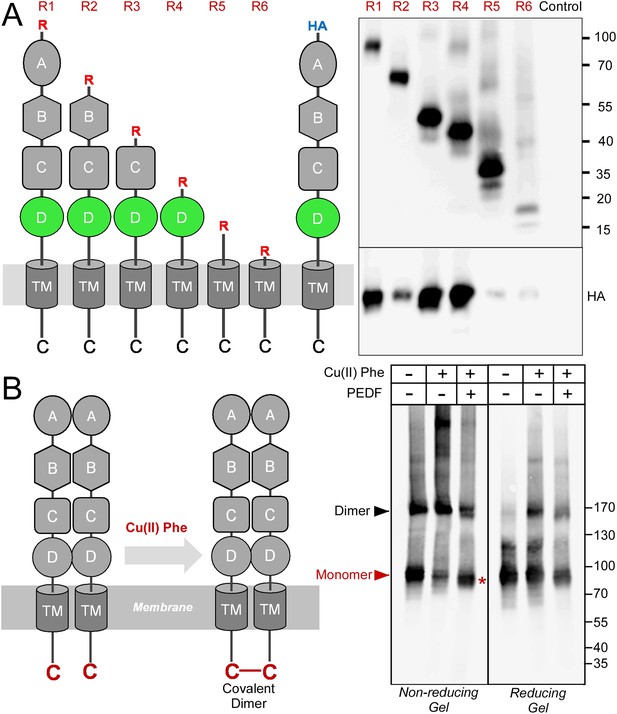
Receptor oligomerization.
(A) PLXDC1 deletion series with a Rim tag following the N-terminal secretion signal were purified together with HA-tagged full length PLXDC1 by purifying the Rim tag (diagrams on the left). Anti-Rim Western is shown on the top and anti-HA Western is shown on the bottom for the elutions. HA-PLXDC1 no longer copurifies if domain D is deleted. (B) Copper phenanthroline [Cu (II) Phe] treatment creates covalent receptor dimer through oxidation of the free cysteine residue on the cytoplasmic tail (schematic diagram on the left). Cu (II) Phe oxidation creates disulfide bond-linked covalent PLXDC1 dimer, as indicated in the Western blot for the receptor. PEDF inhibits dimer formation as shown by increased monomer band on a non-reducing gel after Cu (II) Phe oxidation (red asterisk). The disulfide bond-linked dimers are sensitive to DTT treatment as shown in the reducing gel on the right. Molecular weight markers are in kD.
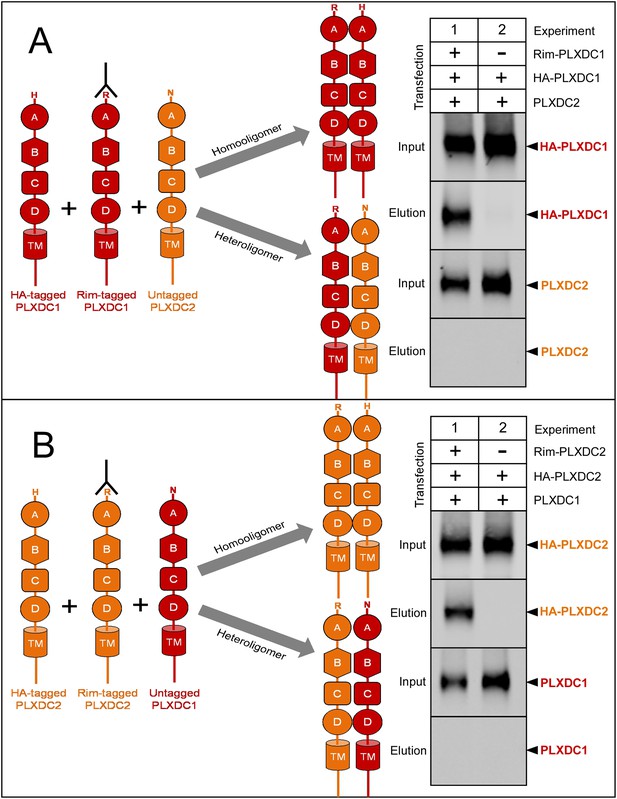
PLXDC1 and PLXDC2 preferentially form homooligomers.
(A) Schematic diagram of experimental design to compare homooligomer and heterooligomer formation of PLXDC1 is shown on the left. Purification of the Rim tag is indicated by an antibody symbol. Anti-Rim purification of Rim-PLXDC1 from cells coexpressing Rim-PLXDC1, HA-PLXDC1 and PLXDC2 leads to HA-PLXDC1 purification, but not PLXDC2 purification (Experiment 1). This experiment suggests that PLXDC1 preferentially forms homooligomers. As a control, HA-PLXDC1 does not get purified in the absence of Rim-PLXDC1 (Experiment 2). Input is the starting material before anti-Rim purification. (B) Schematic diagram of experimental design to compare homooligomer and heterooligomer formation of PLXDC2 is shown on the left. Anti-Rim purification of Rim-PLXDC2 from cells coexpressing Rim-PLXDC2, HA-PLXDC2 and PLXDC1 leads to HA-PLXDC2 purification, but not PLXDC1 purification (Experiment 1). This experiment suggests that PLXDC2 preferentially forms homooligomers. As a control, HA-PLXDC2 does not get purified in the absence of Rim-PLXDC2 (Experiment 2).
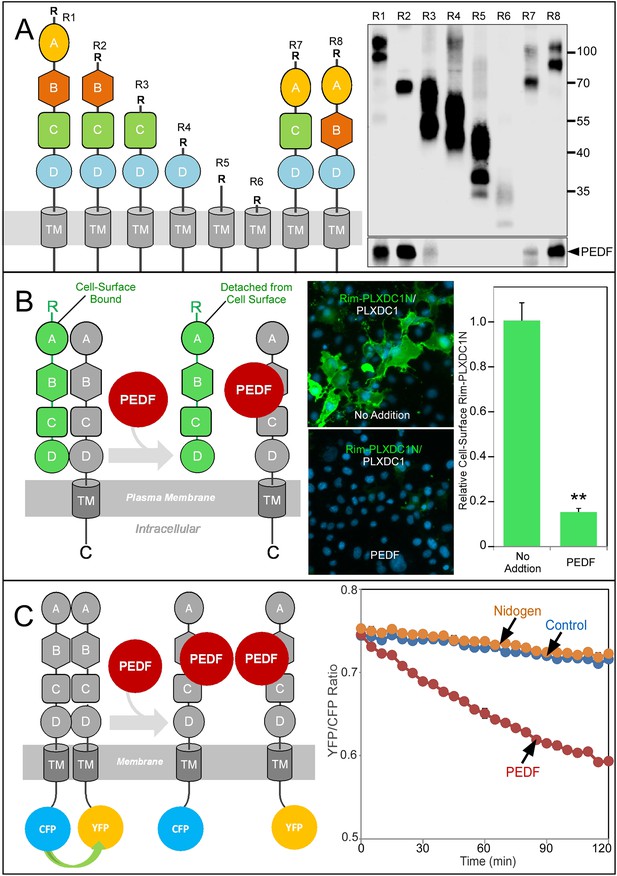
Structure/function and real-time analysis of PEDF/receptor interaction.
(A) PEDF with HA tag after the N-terminal secretion signal is copurified with different deletion mutants of PLXDC1, which are all tagged with a Rim tag following the N-terminal secretion signal (diagrams on the left). Purification of the Rim-tagged proteins with HA-PEDF is shown on the right. The upper Western is the anti-Rim Western and the lower Western is anti-HA Western to detect PEDF. Deletion of domain B largely abolishes the interaction between PLXDC1 and HA-PEDF. (B) An assay to study PEDF-mediated disruption of receptor dimers on the cell surface. Schematic diagram is on the left. PEDF displaces Rim-tagged PLXDC1 extracellular domain (green) bound to cell surface PLXDC1 (gray) to cause detachment of the extracellular domain. Immunostaining of Rim-tagged PLXDC1 extracellular domain, Rim-PLXDC1N, (cotransfected with PLXDC1) on live cell surface is shown in the middle two pictures (green signal). Blue color is nucleic acid stain DAPI. Upper picture: control (no PEDF). Lower picture: PEDF treated. Quantitation of bound Rim-tagged PLXDC1 extracellular domain on the cell surface with or without PEDF treatment is shown on the right. (C) An assay to study PEDF-mediated disruption of receptor dimerization in real time. Schematic diagram of the experimental design is shown on the left. The cytoplasmic tail of PLXDC1 is linked to CFP or YFP, which are in close proximity due to receptor dimerization. PEDF suppresses the FRET signal between CFP and YFP if it disrupts the association of the receptor dimer. Right panel: PEDF, but not a control protein (nidogen) causes a time-dependent decrease in FRET signal between PLXDC1-CFP and PLXDC1-YFP. Both PEDF and nidogen were added at time 0.
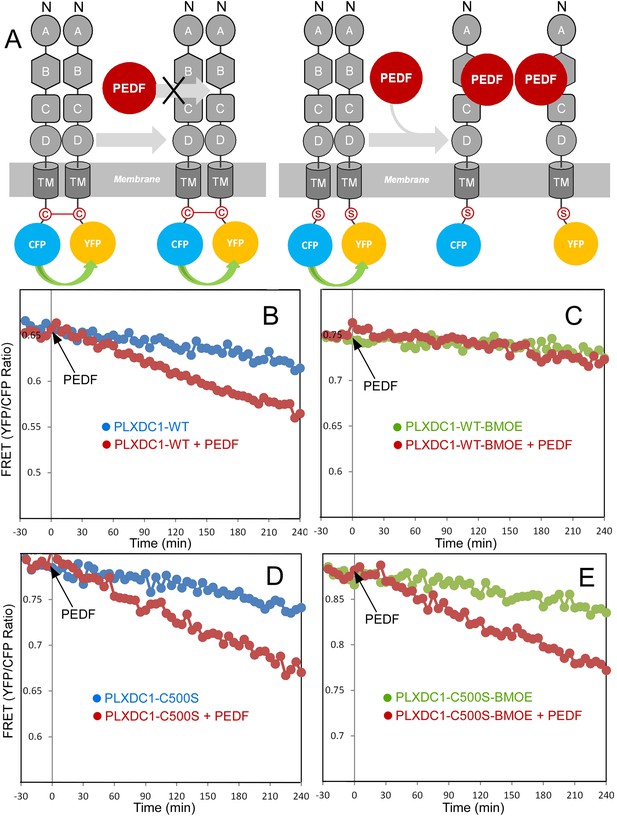
Crosslinking of the terminal cysteine of PLXDC1 prevents PEDF's effect on receptor dimerization.
(A) Schematic diagrams of the experimental design. ‘C’ indicates the cysteine residue located at the C-terminus of PLXDC1. ‘S’ indicates mutation of this residue to serine. (B and C) Crosslinking of the cysteine on the cytoplasmic tail of wild-type PLXDC1 (PLXDC1-WT) by sulfhydryl-specific crosslinker BMOE prevents the PEDF-dependent decrease of FRET signal between PLXDC1-CFP and PLXDC1-YFP. (D and E) BMOE has no effect on PEDF-dependent decrease of FRET signal between PLXDC1-CFP and PLXDC1-YFP if the C-terminal cysteine is mutated to serine (PLXDC1-C500S).





