Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling
Figures
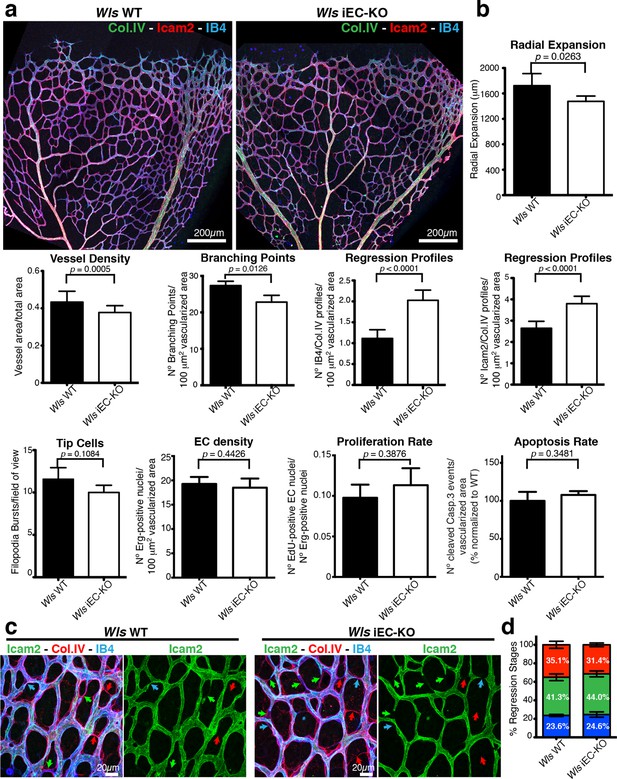
EC-derived Wnt ligands protect against vessel regression.
(a) Overview of retinal vascular plexus of P6 control and Wls iEC-KO mice, labeled for lumen (ICAM2), ECs (IB4) and ECM (Col.IV). (b) Quantification of vascular parameters demonstrating that increased vessel regression is a main feature of Wls iEC-KO in P6 retinas. IB4/Col.IV regression profiles correspond to the number Col.IV-positive segments negative for IB4 staining. ICAM2/Col.IV regression profiles correspond to number of Col.IV-positive vessel segments partially or totally negative for ICAM2 staining. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 6 mice; 3 litters. (c) High magnification images of the vascular plexus of control and Wls iEC-KO mice marked for each stage of vessel regression (stenosis, blue arrows; retraction, green arrow; resolution, red arrows). (d) Quantification of the each specific vessel regression stage in Wls iEC-KO and Wls WT P6 retinas (stenosis, blue bars; retraction, green bars; resolution, red bars). Two-way ANOVA with Sidak multiple comparisons test. Mean +/-SEM; N = 5 mice; 3 litters; N = 288 and N = 398 regression events for Wls WT and Wls iEC-KO mice, respectively.
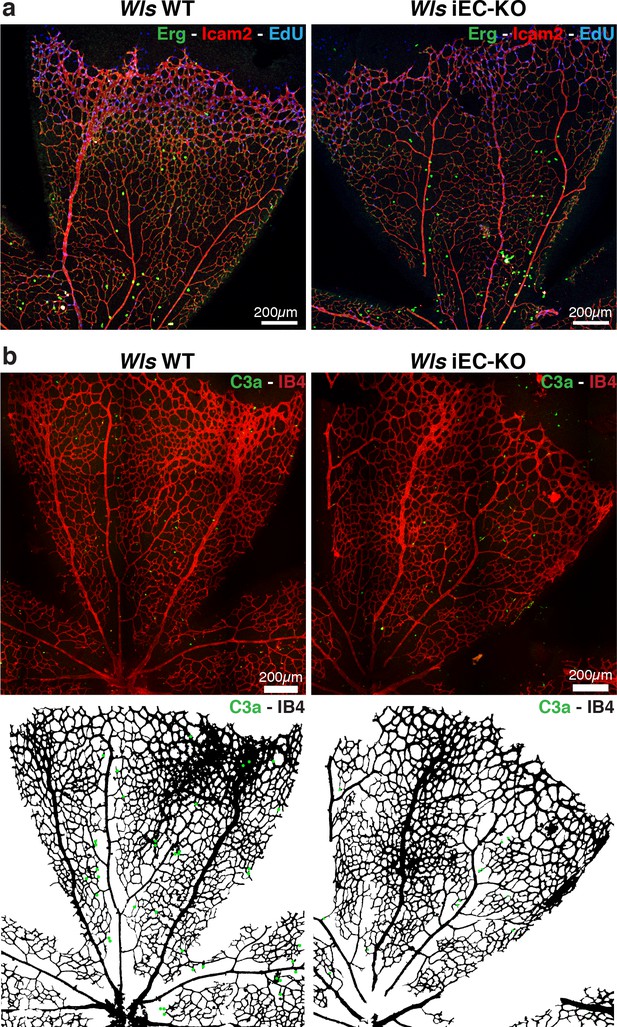
Wls iEC-KO mice show normal proliferation and apoptosis rates.
(a) Representative confocal images of proliferation profile in Wls iEC-KO and Wls WT P6 mouse retinas labeled with ICAM2 (red), endothelial cell nuclei (Erg, green) and EdU (blue). (b) Representative segmentation of confocal images stained for active caspase-3 and blood vessels (IB4) showing distribution of endothelial cells positive for active caspase-3 (green dots) in Wls iEC-KO and Wls WT P6 mouse retinas.
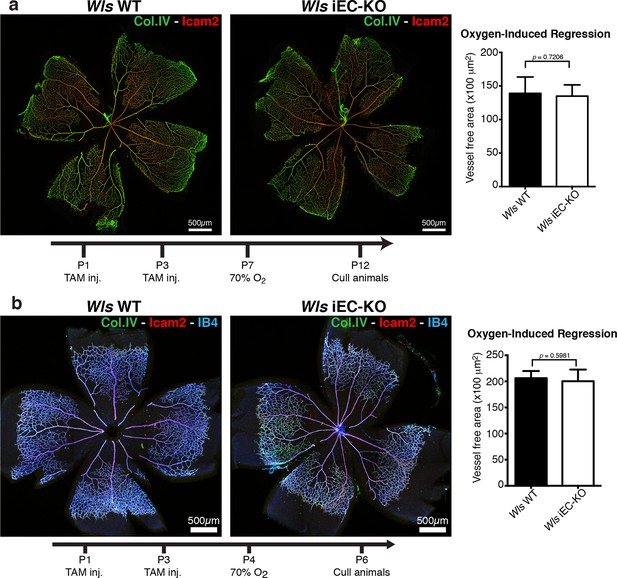
Oxygen-induced vessel regression is not enhanced in Wls iEC-KO mice.
(a) Representative confocal images of Wls iEC-KO and Wls WT P12 mouse retinas under 70% oxygen concentration from P7 until P12, and labeled for vessel lumen (Icam2, red) and extracellular matrix (Col.IV, green). Quantification shows no significant difference in area of vessel obliteration between Wls iEC-KO and Wls WT mouse. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 6 mice; 2 litters. (b) Representative confocal images of Wls iEC-KO and Wls WT P16 mouse retinas under 70% oxygen concentration from P4 until P6, and labeled with vessel lumen (Icam2, red), endothelial cells (IB4, blue) and extracellular matrix (Col.IV, green). Quantification shows no significant difference in area of vessel obliteration between Wls iEC-KO and Wls WT mouse. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 6 mice; 3 litters.
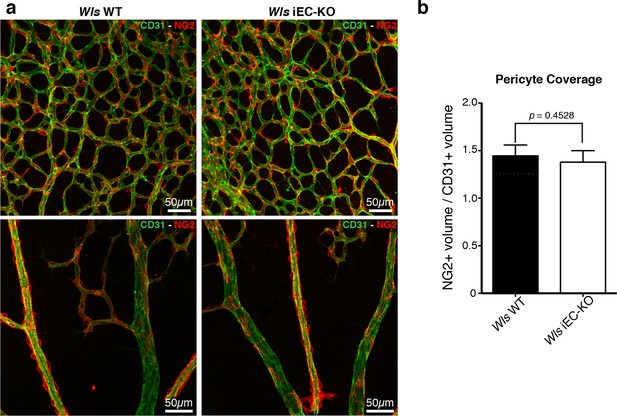
Normal pericytic coverage in Wls iEC-KO.
(a) Overview of retinal vascular plexus of P6 control and Wls iEC-KO mice, labeled for endothelial cells (CD31), and pericytes (Ng2), in capillary plexus (top panels) and main vessels (bottom panels). (b) Quantification of pericyte coverage of retinal blood vessels, showing no significant change between control and Wls iEC-KO in P6 retinas. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 4 mice; 2 litters.
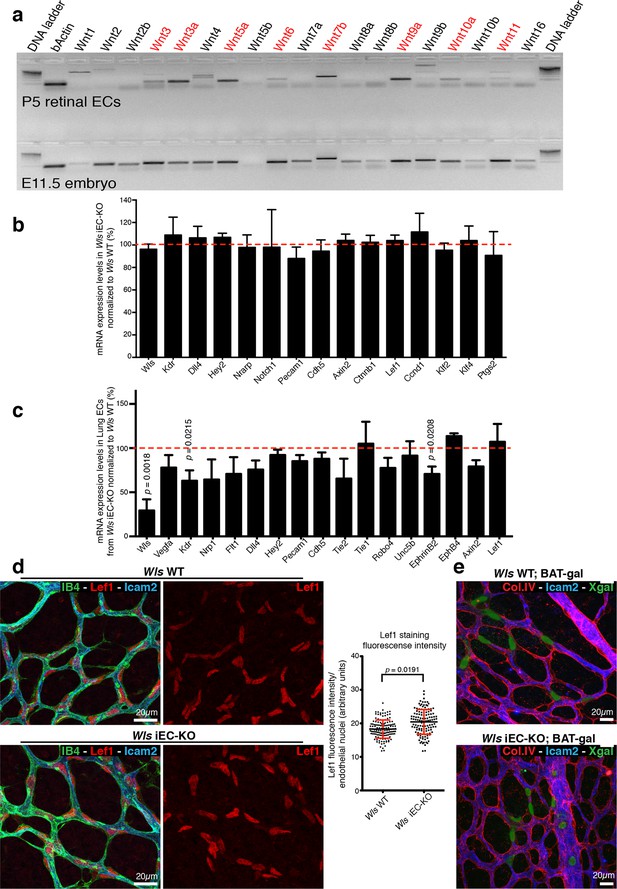
Wls iEC-KO show no significant defects in canonical Wnt signalling.
(a) RT-PCR for all known mouse Wnt ligands using mRNA extracts from isolated retinal endothelial cells of P7 wild-type retinas (red text represents positive bands). (b) Semi-quantitative real-time analysis of mRNA expression levels of different genes in P6 Wls iEC-KO retinas normalized to Wls WT retinas from whole retina extracts. p values from unpaired, two-tailed t-test. Mean +/- SD; N = 4 mice; 2 litters. (c) Semi-quantitative real-time analysis of mRNA expression levels of different genes in P6 Wls iEC-KO normalized to Wls WT from isolated lung endothelial cells. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 3 mice; 2 litters. (d) Lef1 immunostaining and quantification of fluorescence intensity (graph right) in endothelial cells (IB4) from Wls iEC-KO and Wls WT retinas. p values from unpaired, two-tailed t-test. Mean +/- SEM; N = 234 Wls WT cells and N = 128 Wls iEC-KO cells; 4 mice. (e) X-gal staining, indicative of canonical Wnt signalling activation, in Wls iEC-KO; BAT-gal and Wls WT; BAT-gal retinas. No correlation was found with X-gal positive cells and regression profiles (visualized by ECM (Col.IV) and lumen (Icam2) stainings).
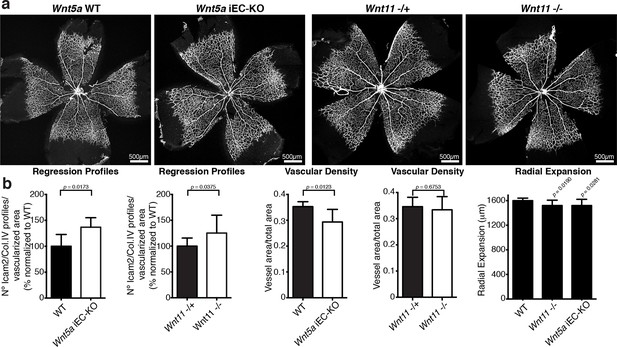
Characterization of vascular parameters in Wnt5a iEC-KO and Wnt11 KO retinas.
(a) Overview of retinal vascular plexus of P6 Wnt5a iEC-KO, Wnt11 KO, and corresponding control mice, labeled with ECM (Col.IV, grey). (b) Quantification of different vascular parameters in P6 retinas on the different mouse strains. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 5 mice; 3 litters.
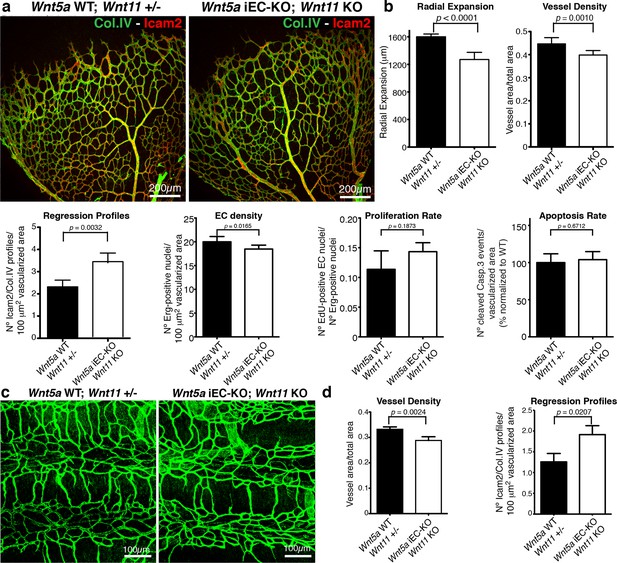
Non-canonical Wnt signalling regulates vessel regression.
(a) Overview of retinal vascular plexus of control and compound Wnt5a iEC-KO; Wnt11 KO mice, labelled with lumen (Icam2) and ECM (Col.IV) markers. (b) Quantification of different vascular parameters showing increased vessel regression in Wnt5a iEC-KO; Wnt11 KO retinas. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 7 mice; 3 litters. (c) Overview of trachea vascular plexus of P6 Wnt5a iEC-KO; Wnt11 KO and control mice labelled for CD31 (green). (d) Quantification of vessel density and regression profiles in the trachea of Wnt5a iEC-KO; Wnt 11 KO and WT P6 mice. p values from unpaired, two-tailed t-test. Mean +/-SEM; N = 4 mice; 2 litters.
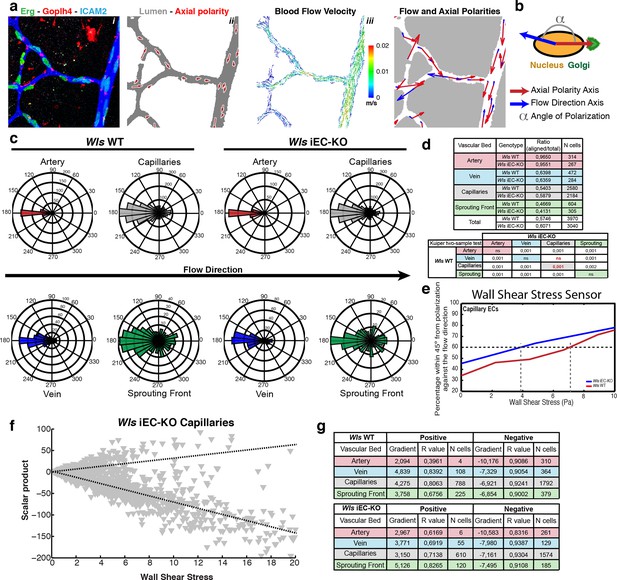
Endothelial-derived Wnt ligands modulate endothelial polarization in response to wall shear stress.
(a) Example of axial polarity of an Wls iEC-KO P6 retina, labeled for EC nuclei (Erg), lumen (Icam2) and Golgi (Golph4) (i), corresponding image segmentation of the vascular plexus with axial polarity vectors in red (ii), flow pattern simulation of the selected area (iii), and correlation between axial polarity and blood flow direction at the endothelial nuclear position (iv). (b) Representation of the principle of angle calculation between axial and flow polarities of endothelial nuclei in (a), highlighting the lumen of blood vessels (grey), and the axial polarity of all ECs (red arrows). (c) Analysis of the endothelial axial polarity angle in the main vessels, relative to predicted blood flow direction by the rheology in silico model in Wls WT and Wls iEC-KO mice (n = 3 retinas). (d) Quantitative analysis of the percentage of ECs polarized at 180°(± 45°) degrees compared to the flow direction in the different vascular beds of Wls WT and Wls iEC-KO mice (n = 3 retinas). (e) Correlative analysis of wall shear stress and EC polarization in the capillary vascular bed of Wls WT and Wls iEC-KO mice. (f) Representative graph showing the distribution of scalar products in function to wall shear stress levels for ECs from Wls iEC-KO capillaries. Scalar product corresponds to the product between length of the axial polarity vector and the cosine of the angle between the axial polarity vector and the flow direction vector. (g) Linear regression analysis of positive (polarized with flow) and negative (polarized against the flow) scalar product points for each endothelial cell nucleus. Gradient, R-value and number of cells analyzed for each vascular bed and genotype are shown. N = 3 retinas.
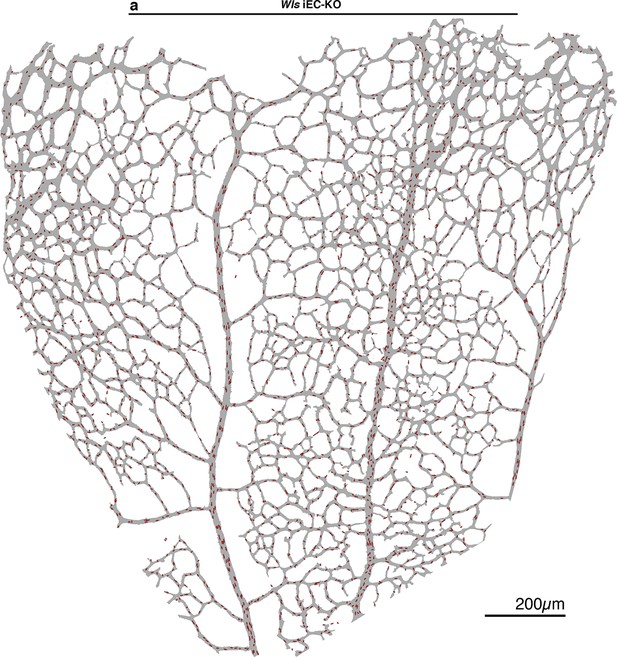
Endothelial polarization patterns in Wls iEC-KO.
(a) Image segmentation of the vascular plexus of an Wls iEC-KO mouse retina, highlighting the lumen of blood vessels (grey) and the nucleus-to-golgi (axial) polarity of all endothelial cells (red arrows).
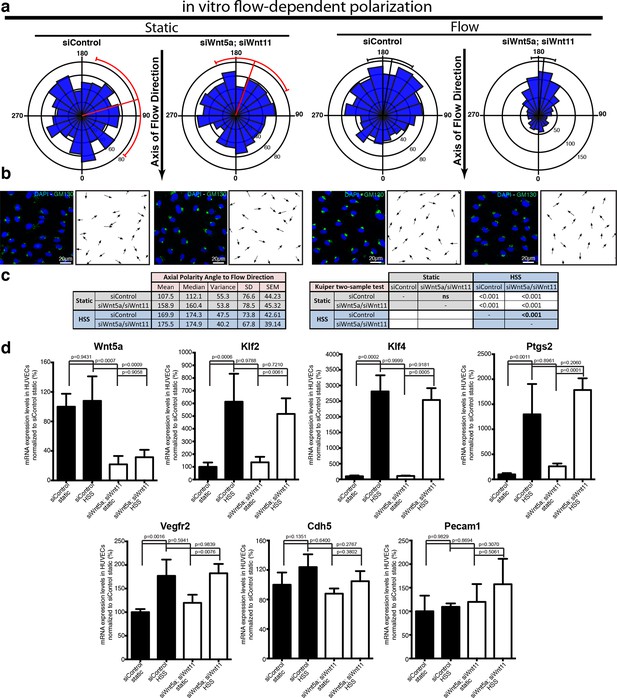
Non-canonical Wnt signalling modulates flow-induced polarity but not flow-induced transcriptional gene expression response.
(a) Rose-plot representation of axial polarity of ECs treated with Control or Wnt5a and Wnt11 specific siRNAs in static conditions or in response to 2 Pa flow in a microfluidic device (n = 3 independent experiments). Line running from the center represents the Mean of the dataset. Arcs extending to either side represent the 95% confidence limits of the mean (red means not significantly polarised; black means significantly polarised). (b) Representative images of endothelial cell polarity in flow chamber stained for nuclei (Dapi, Blue) and Golgi apparatus (GM130, green), and corresponding axial polarity vectors (black arrows). (c) Quantitative analysis of EC polarization related to the flow direction in the microfluidic device. p values from non-parametric two-tailed Kuiper’s test. (d) Semi-quantitative real-time analysis of mRNA expression levels of different genes in Control or Wnt5a and Wnt11 specific siRNAs in static or stimulated with 2 Pa conditions in a microfluidic device. p values from one-way ANOVA with multiple comparisons. Mean +/-SD; N = 3 independent experiments.
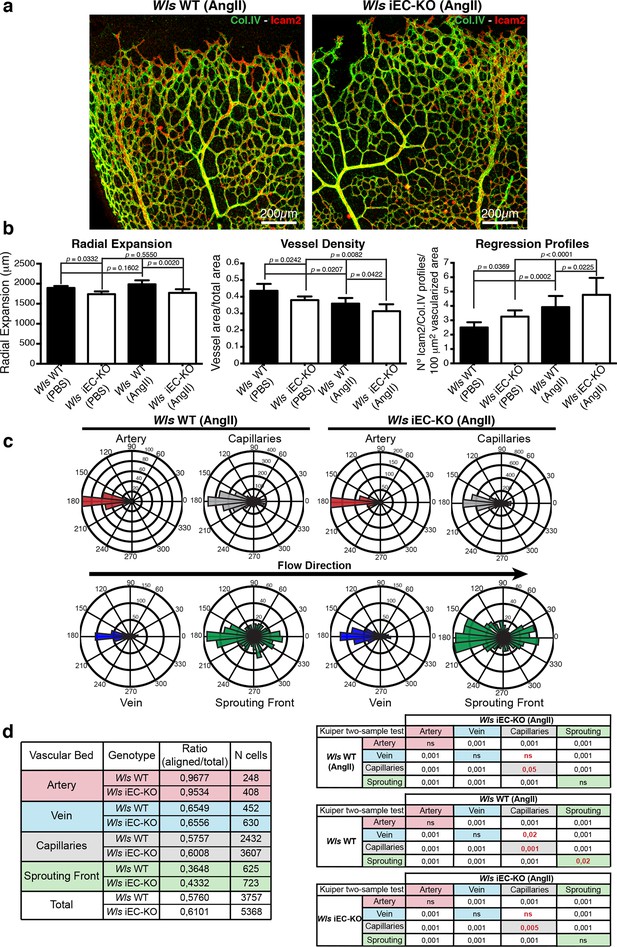
Systemic Angiotensin-II treatment accelerates vessel regression independent of Wnt signalling.
(a) Overview of retinal vascular plexus of P7 control and Wls iEC-KO mice treated with angiotensin II, labelled for lumen (Icam2) and ECM (Col.IV). (b) Quantification of radial expansion, vessel density and regression profiles in angiotensin II-treated and PBS-treated control retinas. p values from one-way ANOVA with Holm-Sidak’s multiple comparison test. Mean +/-SEM; N = 4 mice; 3 litters. (c) Analysis of the endothelial axial polarity angle in the main vessels, correlated to predicted blood flow direction by the rheology in silico model in Wls WT and Wls iEC-KO mice treated with angiotensin II. (d) Quantitative analysis of the percentage of ECs polarized at 180°(± 45°) degrees compared to the flow direction in the different vascular beds of Wls WT and Wls iEC-KO mice with and without angiotensin II treatment (n = 3 retinas).
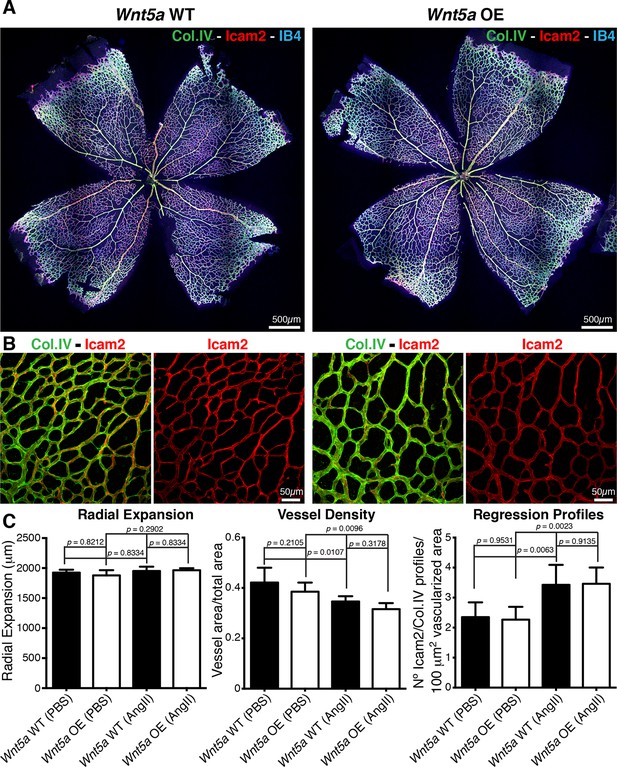
Overexpression of Wnt5a in endothelial cells does not inhibit vessel regression.
(a) Overview of retinal vascular plexus of P6 endothelial-specific Wnt5a GOF and corresponding control mice, labeled for ECM (Col.IV, green), blood vessels (IB4, blue) and vascular lumen (Icam2, red). (b) Higher magnification of retinal vascular plexus of P6 endothelial-specific Wnt5a GOF and corresponding control mice, labeled for ECM (Col.IV, green), blood vessels (IB4, blue) and vascular lumen (Icam2, red). (c) Quantification of vascular parameters showing no significant differences between Wnt5a GOF and corresponding control retinas, with indicated treatments. p values from one-way ANOVA with Holm-Sidak’s multiple comparison test. Mean +/-SEM; N = 4 mice; 2 litters.
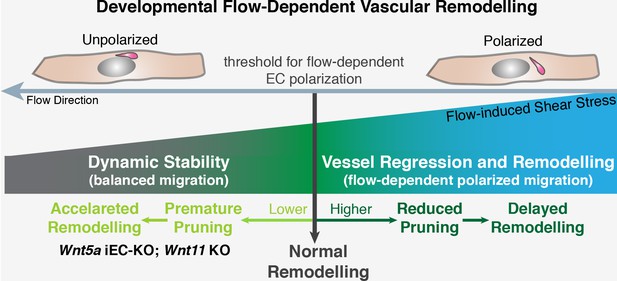
Working model for non-canonical Wnt signalling regulation of flow-induced remodeling.
Initiation of the vascular remodeling program is dependent on the level of wall shear stress (threshold) to which ECs robustly polarize against the flow direction. Below the threshold, ECs movements in the immature plexus are balanced by countermovements of adjacent ECs maintaining vessel connections, in a state of dynamic stability. When exposed to shear stress levels above the response threshold, ECs react by polarizing and migrating against the flow direction, triggering vessel regression and vessel remodeling. Signaling pathways influencing the threshold for flow-dependent EC polarization can delay remodeling (raising the threshold) or induce premature remodeling (lowering the threshold), as in the case of deficient non-canonical Wnt signaling.
Tables
Tie2-Cre Wlsfl/fl embryos die at mid-gestation. Table showing number of embryos collected at the specified embryonic time point post-coitum (E) and at birth. Relative frequency each genotype of embryos/pups is shown as percentage.
| ♂ Tie2-Cre::Wls fl/wt X ♀ Wls fl/fl | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | E12.5 | % | E13.5 | % | E16.5 | % | Adults | % |
| Wlsfl/fl | 6 | 0.25 | 4 | 0.29 | 7 | 0.41 | 20 | 0.38 |
| Wlsfl/wt | 5 | 0.21 | 4 | 0.29 | 4 | 0.24 | 13 | 0.25 |
| Wlsfl/fl::Cre+ | 6 | 0.25 | 4 | 0.29 | 4 (3 dead) | 0.24 | 0 | 0.00 |
| Wlsfl/wt::Cre+ | 7 | 0.29 | 2 | 0.14 | 2 | 0.12 | 20 | 0.38 |
Additional files
-
Supplementary file 1
List of antibodies used in the immunochemistry and immunofluorescence studies
- https://doi.org/10.7554/eLife.07727.017
-
Supplementary file 2
List PCR primers used in Wnt ligand gene expression profiling of isolated retinal endothelial cells
- https://doi.org/10.7554/eLife.07727.018
-
Supplementary file 3
List qPCR Taqman primers used in gene expression experiments.
- https://doi.org/10.7554/eLife.07727.019





