Matrix metalloproteinase 14 is required for fibrous tissue expansion
Figures
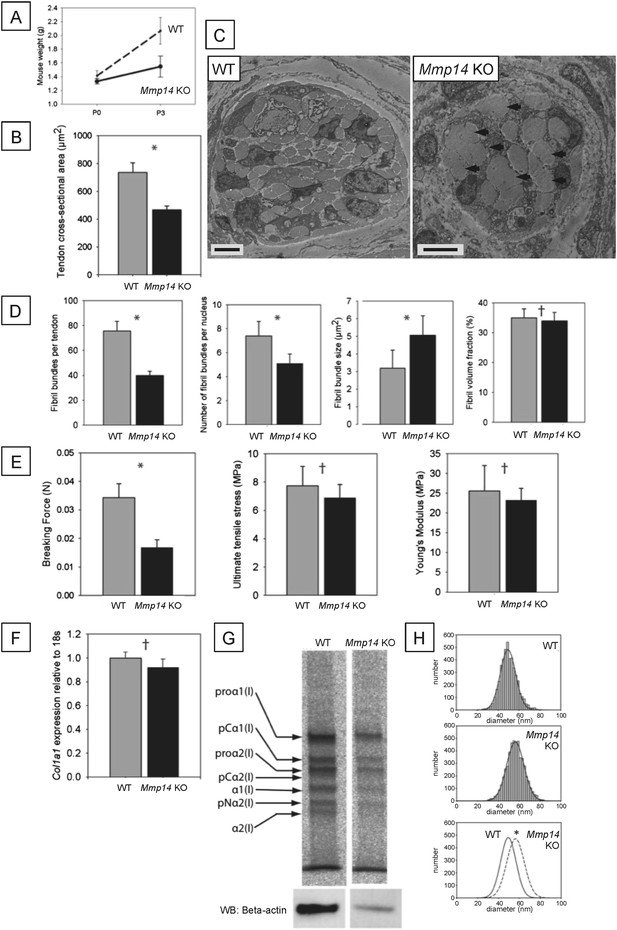
Neonatal Mmp14-deficient mice have small and weak tendons.
(A) Weight of wild type (WT) and Mmp14 knockout (Mmp14 KO) littermates at birth (P0) and at 3 days postnatal (P3). (B) The cross-sectional transverse area of P0 Mmp14 KO tendons is significantly smaller than WT tendons. (C) TEM images of P0 tail tendon demonstrate that KO tendons are smaller and show dysmorphic, enlarged bundles of collagen fibrils (arrowhead). Scale bars 5 μm. (D) KO tendons have fewer, larger fibril bundles, but the FVF is not different to WT tendons. (E) KO tendons are weaker than WT tendons but have normal mechanical properties after adjusting for differences in size. (F) Analysis of Col1a1 mRNA by qPCR in P0 tendons revealed no difference in gene expression. (G) 14C-proline labeling of collagen demonstrated normal collagen processing at P7 in WT and KO tail tendon. (H) Fibril diameter distributions of KO and WT tail tendon at P0 revealed significantly increased fibril diameters in KO mice. Bars show SEM. *p < 0.05, †p > 0.05 (t-tests).
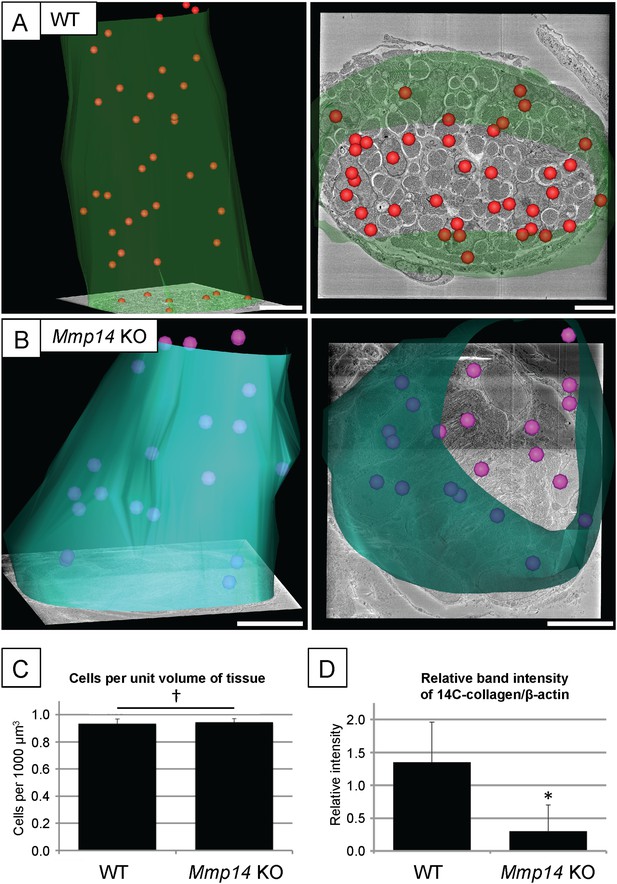
Cell number and type I collagen synthesis in neonatal WT and Mmp14 KO tail tendon.
3D reconstruction from SBF-SEM analysis of P0 (A) WT and (B) Mmp14 KO tendons. Green/turquoise, outline of the tendon. Red/pink spheres, cell nuclei. Scale bars 10 µm. (C) Estimated mean cell number per unit volume of tissue shows that the absence of MMP14 does not affect cell number at birth. Bars show SEM. †p > 0.05 (t-test). (D) Densitometry data for P7 tail tendons were labeled with 14C-proline for 1 hr and separate extracellular and intracellular extracts prepared as described (Canty et al., 2004). A significant decrease in [14C]-collagen relative to β-actin (detected by western blotting) in the intracellular extracts was observed in the KO samples (*p < 0.01, t-test).
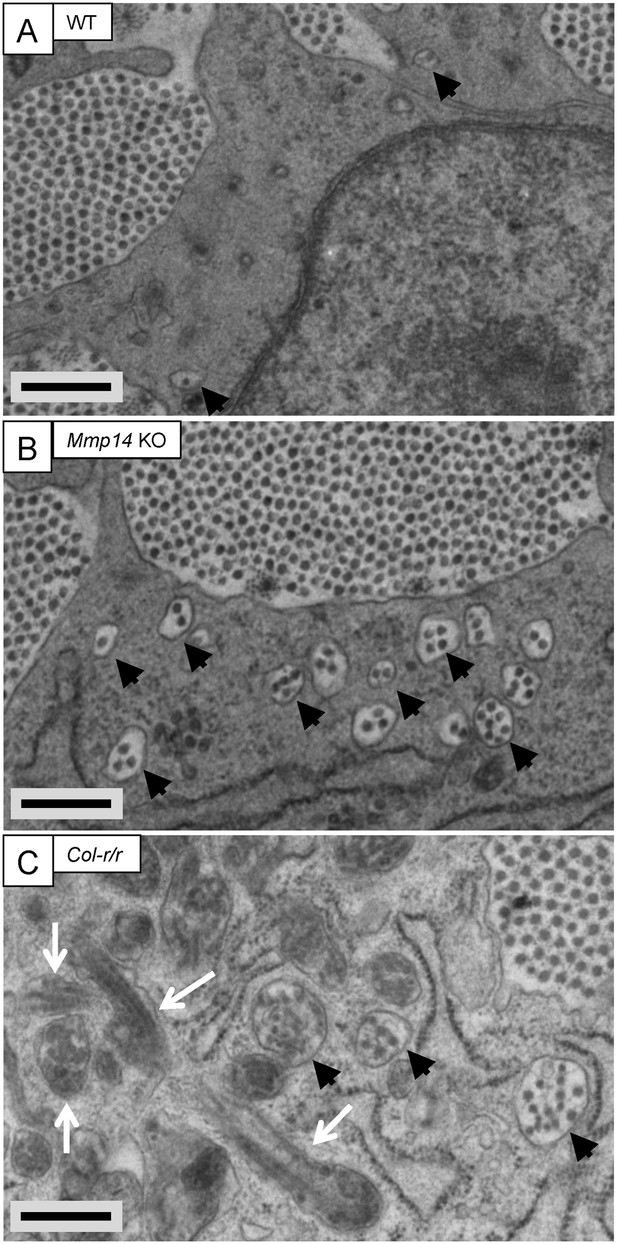
Fibricarrier analysis of wild-type, Mmp14 KO, and Col-r/r embryonic tail tendon.
Tail tendons at E15.5 of development from (A) wild-type, (B) Mmp14 KO, and (C) Col-r/r mice. Black arrowhead, recessed fibripositor (electron lucent)-containing collagen fibrils. White arrow, enclosed electron-dense compartment. Scale bars 500 nm.
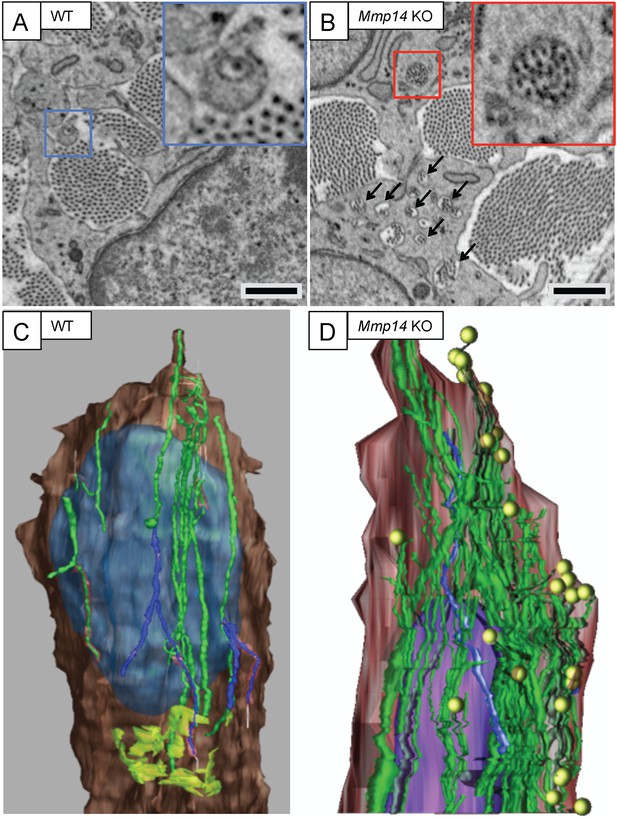
Mmp14-deficient mice have prominent recessed fibripositors.
SBF-SEM of (A) WT and (B) Mmp14 KO tendons at E15.5 showing fibripositors in WT tendons (blue box) and intracellular collagen fibril-containing compartments in Mmp14 KO tendons (red box; arrowheads). Scale bars 1 µm. 3D reconstruction of SBF-SEM data from (C) WT and (D) Mmp14 KO tendon cells. Brown, cytoplasm. Purple/blue, nucleus. Green, internal collagen fibril compartments. Yellow circles, fibril ends. Lime green (in WT only), Golgi apparatus.
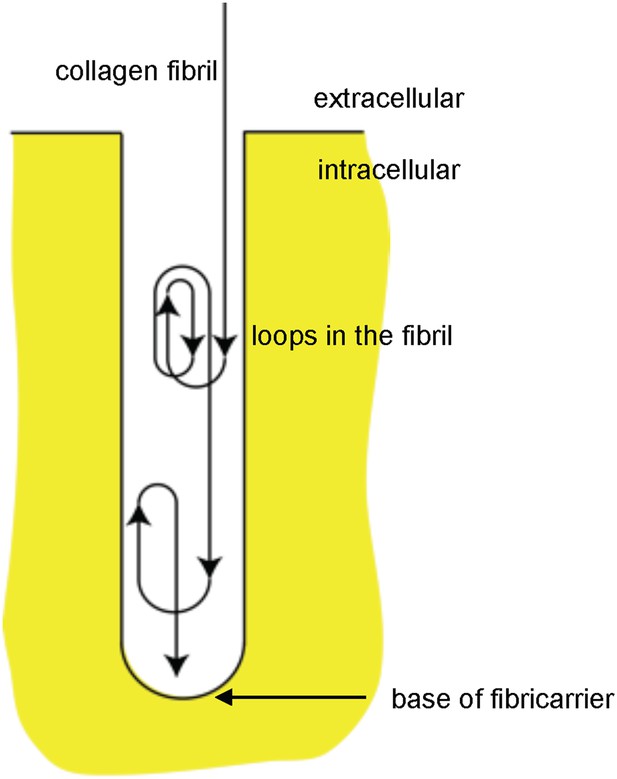
Schematic showing looping of collagen fibrils in recessed fibripositors.
Diagrammatic representation of a recessed fibripositor with looping of a collagen fibril. Not drawn to scale.
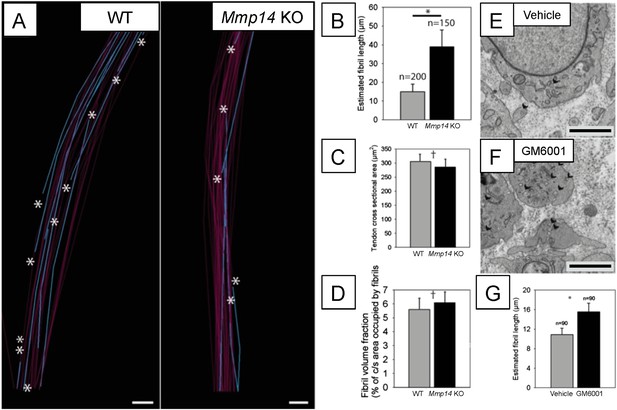
Deficiency in MMP14 activity results in fewer collagen fibrils.
(A) 10 µm-deep (z-axis) slices of 3D reconstructions of SBF-SEM data taken from of WT and Mmp14 KO embryonic tendons at E15.5 showing collagen fibrils (blue) with a tip (marked by asterisks) found within the volume. Purple fibrils passed through the volume and so did not have tips in the reconstruction. Scale bars 500 nm. (B) Quantification of mean fibril length based on the number of tips identified shows that E15.5 WT fibrils are shorter than fibrils in age- and anatomical position-matched tail tendons from KO mice (308 and 266 fibrils tracked, respectively). (C) Tendon cross-sectional area and (D) FVF are not different at E15.5 KO tendons. (E, F) Electron microscopy of tendon-like constructs cultured in the presence of MMP inhibitor GM6001 (10 µM in 0.1% DMSO) show increased number of recessed fibripositors (arrowheads) compared to vehicle control. Scale bars 1 µm. (G) Increase in calculated mean fibril length in GM6001-treated tendon-like constructs. Bars show SEM. *p < 0.05, †p > 0.05 (t-tests).
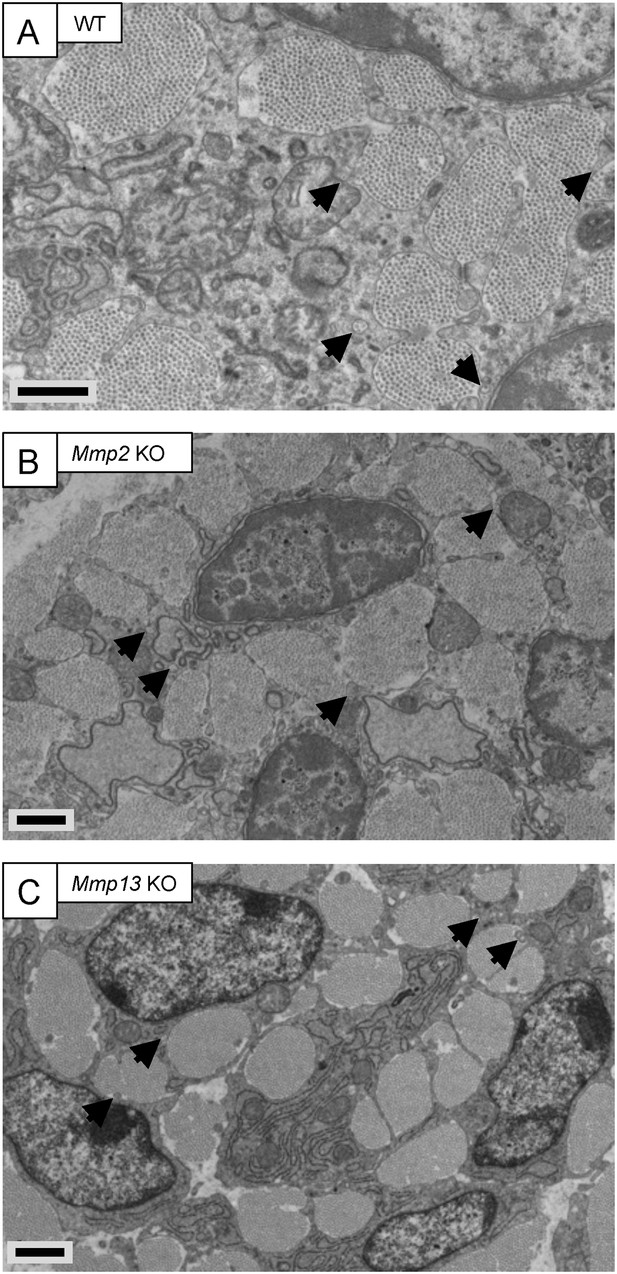
Embryonic tendons deficient in Mmp2 or Mmp13 do not have overt tendon phenotypes.
Electron microscopy images of embryonic tendons from (A) WT, (B) Mmp2 KO, and (C) Mmp13 KO mice. Arrowheads indicate the locations of fibripositors. Scale bars 1 µm.
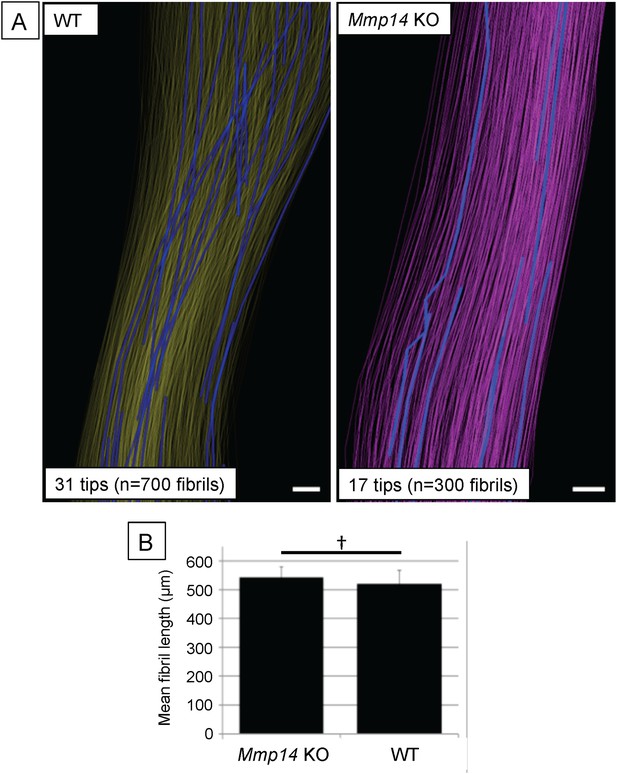
Deficiency in MMP14 activity results in fewer collagen fibrils tips at P0.
(A) 3D reconstruction from SBF-SEM analysis of P0 WT and Mmp14 KO tendons. Scale bars 2 µm. (B) Estimated mean fibril length 645 ± 14 µm WT and 674 ± 24 µm Mmp14 KO shows that the absence of MMP14 does not affect fibril length at birth. Bars show SEM. †p > 0.05 (t-test).
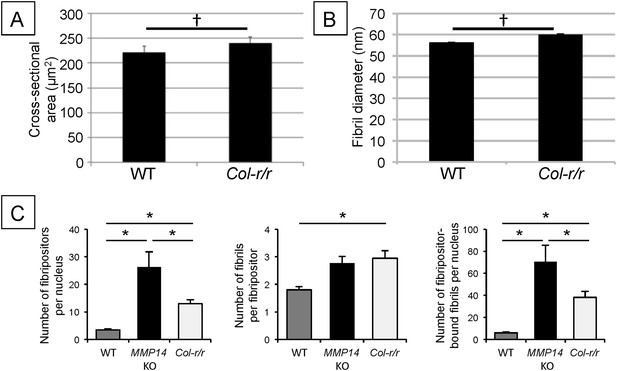
Quantitative analysis of Col-r/r embryonic tendons.
Measurement of tendon cross-sectional area (A) and collagen fibril diameter (B) shows no significant difference between WT and Col-r/r mice at E15.5. †p > 0.05 (t-tests). (C) Quantitation of fibril-containing carriers in WT, Mmp14 KO, and Col-r/r embryonic tenocytes. *p < 0.05 (one-way ANOVA). Bars show SEM.
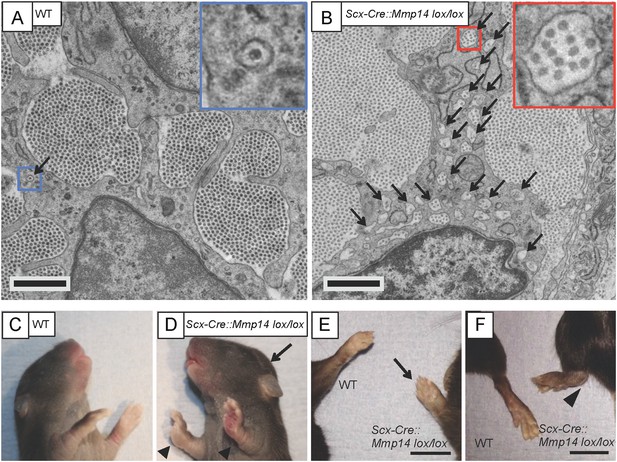
Scx-Cre::Mmp14 lox/lox mice have limb and skeletal deformities.
Tail tendons from littermates at P0 from (A) WT and (B) Scx-Cre::Mmp14 lox/lox mice show Mmp14-null tendons have multiple fibripositors-containing multiple fibrils (red box) than fibripositors in WT tendons (blue box). Black arrowhead, recessed fibripositor (electron lucent)-containing collagen fibrils. Scale bars 500 nm. (C) Control pups at P8 showed normal limb development but (D) Scx-Cre::Mmp14 lox/lox littermates show dorsiflexion of their limbs (arrowhead) and dome-shaped skull (arrow). Adult (7 week old) Scx-Cre::Mmp14 lox/lox mice have (E) enlarged paws (open arrow) and (F) extreme dorsiflexion of hind limbs (arrowhead) compared to control littermates. Scale bars 1 cm.
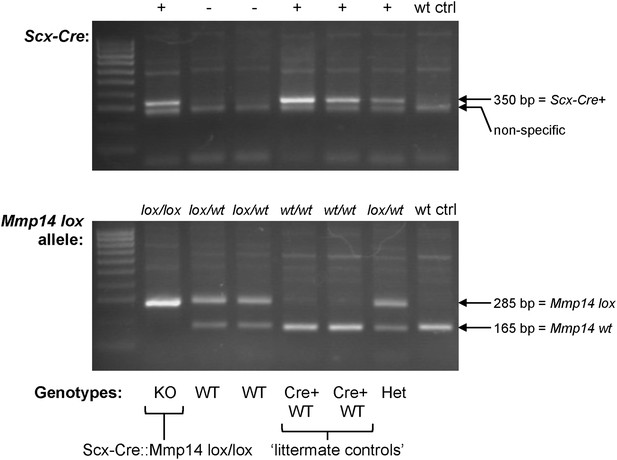
Genotyping the Scx-Cre::Mmp14 lox/lox colony.
A typical genotyping result to identify Scx-Cre::Mmp14 lox/lox mice. Wt ctrl, control wild-type DNA. Het, heterozygous (lox/wt, Cre+). KO, homozygous knockout (lox/lox, Cre+). WT, wild-type (no Cre). Cre+ WT, Cre-expressing WT (wt/wt, Cre+) used as controls.
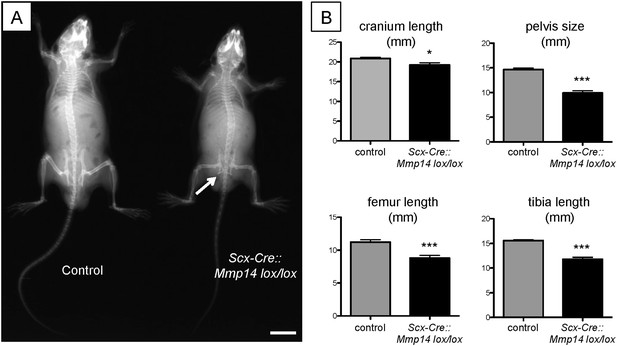
Adult Scx-Cre::Mmp14 lox/lox mice have skeletal deformities.
(A) Representative x-ray shows adult (7 weeks old) Scx-Cre::Mmp14 lox/lox mice are smaller than control littermates and have reduced bone density. White arrow indicates hip dysplasia. Scale bars 1 cm. (B) 7-week-old Scx-Cre::Mmp14 lox/lox mice have shorter cranium length, smaller pelvis, and shorter long bones. Bars show SEM. *p = 0.0105; ***p < 0.001 (t-tests; 12 control, 8 Scx-Cre::Mmp14 lox/lox).
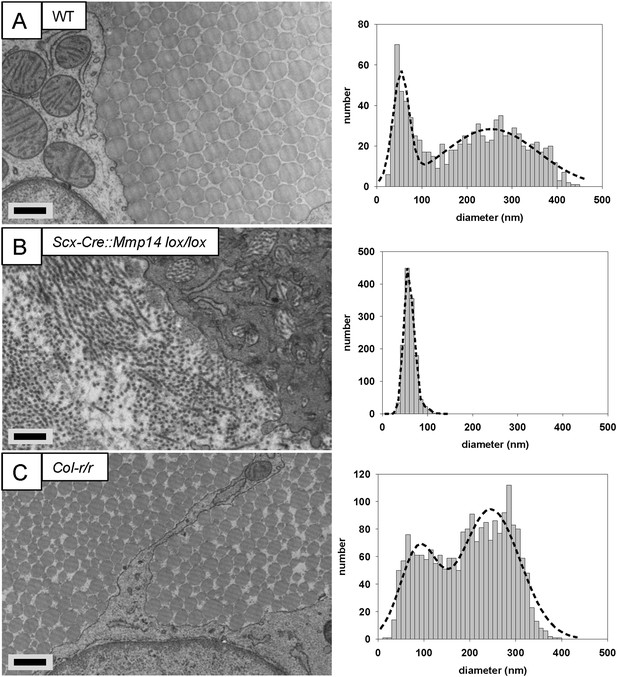
Deficiency in MMP14 activity inhibits bimodal fibril diameter distribution in tendons from adult mice.
Tail tendons from 7 week-old (A) WT, (B) Scx-Cre::Mmp14 lox/lox, and (C) Col-r/r mice. Larger diameter fibrils can be observed in the ECM of WT and Col-r/r postnatal tendons but only narrow diameter fibrils are observed in Mmp14-deficient postnatal tendons. Scale bars 500 nm.
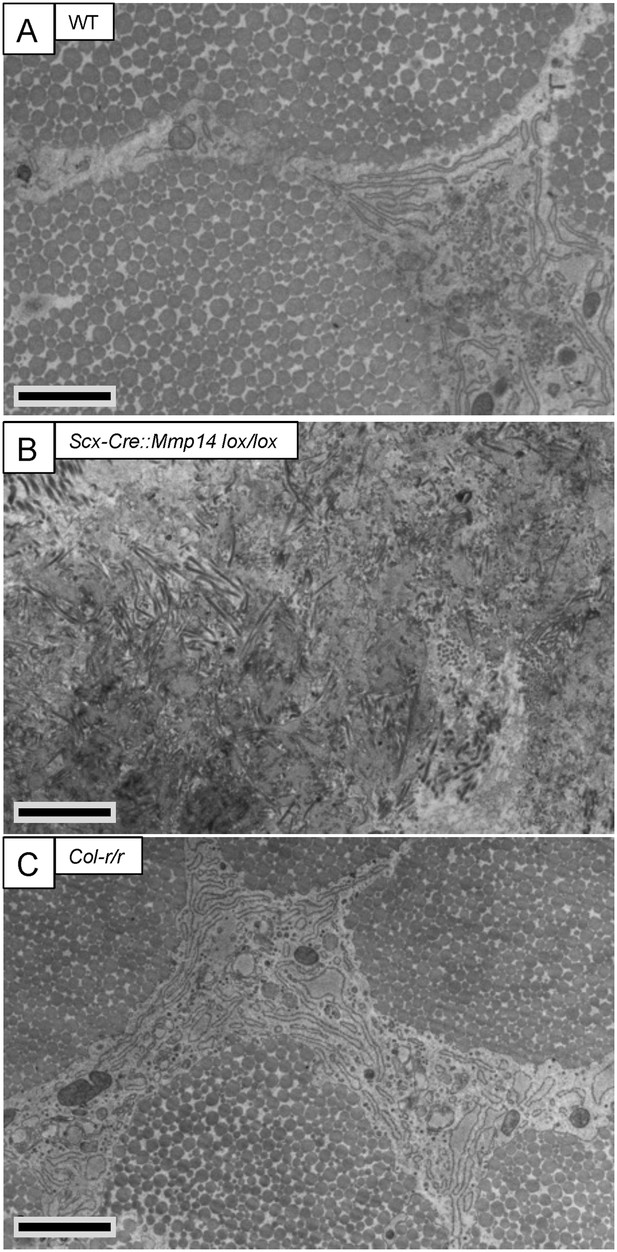
Cleavage of the ¾-¼ collagen-I site is not required for release of fibrils in tendons from adult mice.
Electron microscopy images of tendons from 7-week-old (A) WT, (B) Scx-Cre::Mmp14 lox/lox, and (C) Col-r/r mice. Larger diameter fibrils occur in the ECM of WT and Col-r/r postnatal tendons but only narrow diameter fibrils occur in Mmp14-deficient postnatal tendons. Scale bars 2 µm.
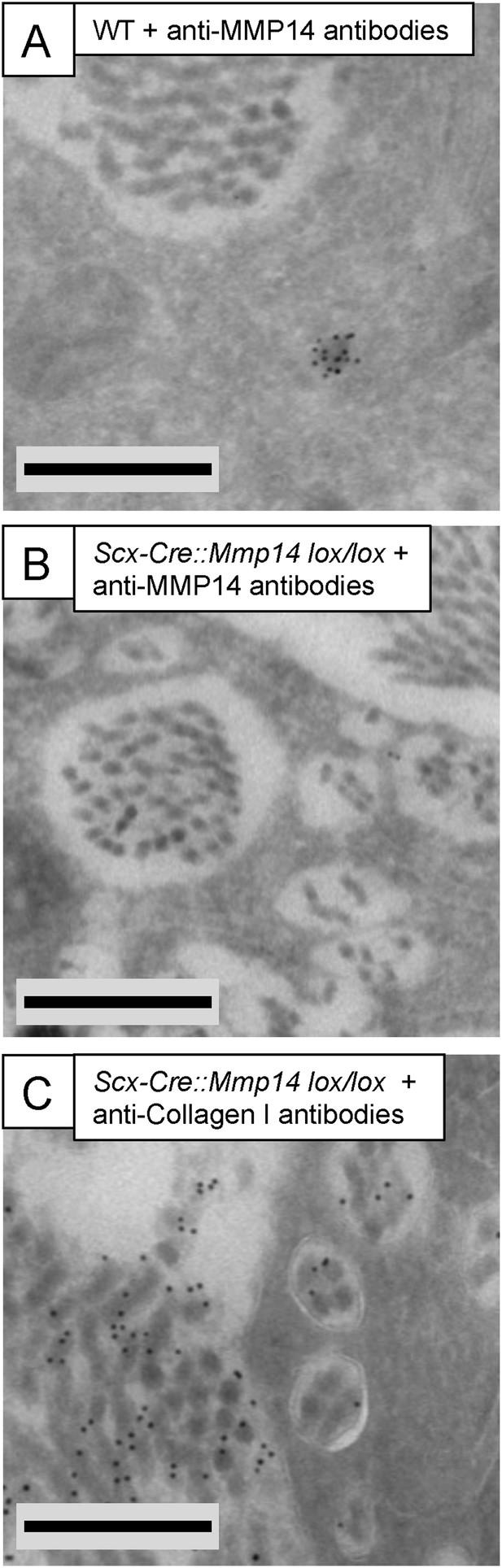
Immuno-electron microscopy of Scx-Cre::Mmp14 lox/lox tendon.
(A) WT and (B) Scx-Cre::Mmp14 lox/lox tail tendon from P7 (7 days) mice were labeled with anti-MMP14 antibody. (C) Scx-Cre::Mmp14 lox/lox tendon labeled with anti-collagen-I antibody. Scale bars 500 nm.
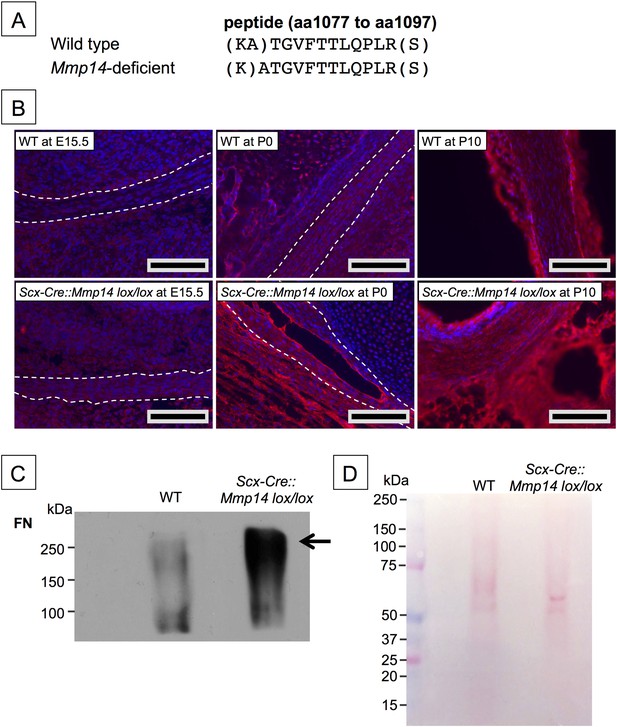
Elevated FN in Mmp14-deficient tendons.
(A) Sequence of a unique semi-tryptic peptide of FN identified in neonatal (P7-10) WT tendon and the sequence of the corresponding peptide from Mmp14-deficient tendons without the additional Ala(1078)-Thr(1079) cleavage. (B) Immunofluorescence analysis of FN in tendons of WT and Scx-Cre::Mmp14 lox/lox mice at E15.5, P0, and P10 of development. Scale bars 200 µm. (C) Western blot analysis of P7 WT and Scx-Cre::Mmp14 lox/lox tendons show elevated FN in Mmp14-deficient tendons. (D) Ponceau S-stained membrane shows equivalent extractability of WT and Scx-Cre::Mmp14 lox/lox tendons.
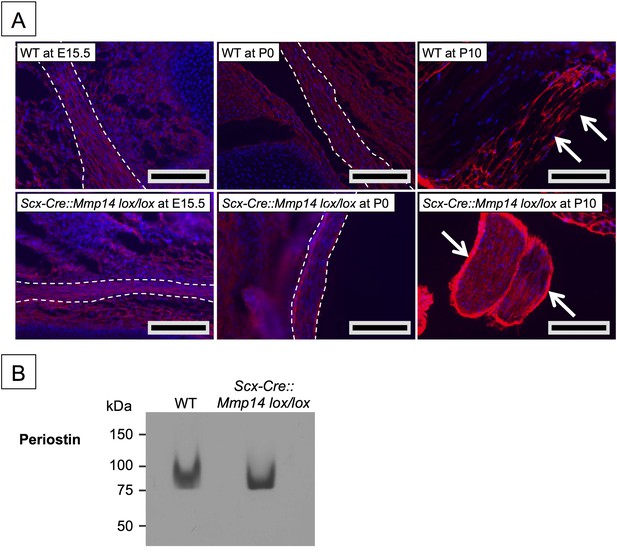
Elevated periostin levels only in postnatal Scx-Cre::Mmp14 lox/lox tendons.
(A) Immunofluorescence analysis of periostin in tendons of WT and Scx-Cre::Mmp14 lox/lox mice at E15.5, P0, and P10 of development show accumulation of periostin in epithelium (white arrows) of Scx-Cre::Mmp14 lox/lox tendons. Scale bars 200 µm. (B) Western blot analysis of P7 WT and Scx-Cre::Mmp14 lox/lox Achilles tendons show similar levels of periostin in Mmp14-deficient tendons.
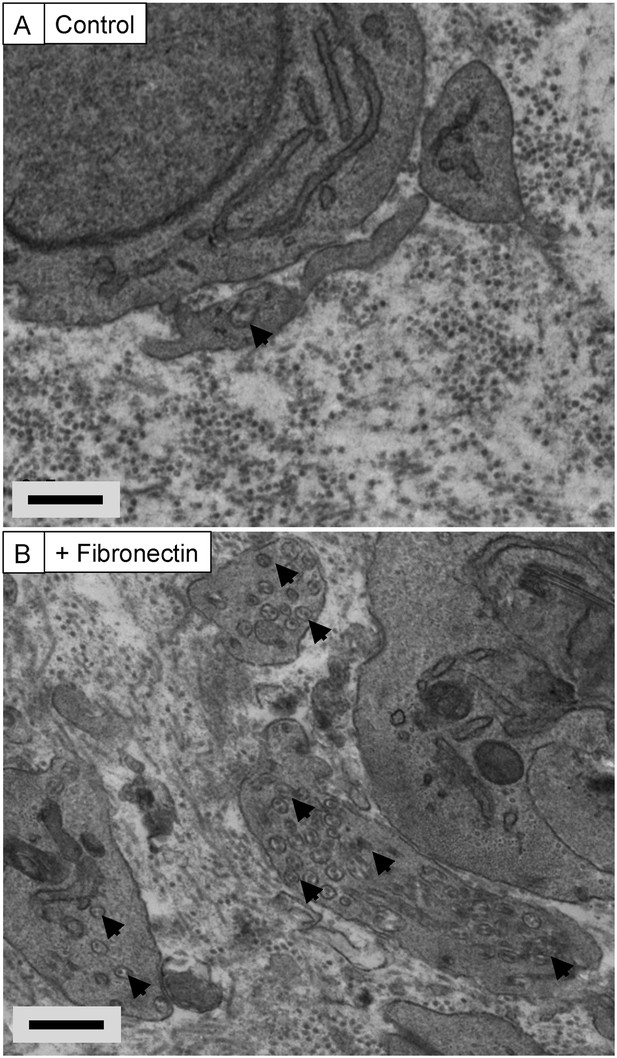
Exogenous FN induces recessed fibripositors in tendon-like constructs.
Transmission electron microscopy of tendon-like constructs in the absence (A) and presence (B) of 200-µg/ml human plasma fibronectin. Arrowheads show recessed fibripositors, which were abundant in the treated constructs. Scale bars 500 nm.
Videos
Step-through video generated from SBF-SEM images of E17.5 embryonic WT mouse-tail tendon.
z-depth is 100 µm. Scale bar 2 µm.
Step-through video generated from SBF-SEM images of E17.5 embryonic Mmp14 KO mouse-tail tendon.
z-depth is 100 µm. Scale bar 2 µm.
Step-through video generated from SBF-SEM images of E17.5 embryonic Col-r/r mouse-tail tendon. z-depth is 100 µm.
Scale bar 2 µm.
Additional files
-
Supplementary file 1
LC-MS/MS peptide identity and counts. Editable Microscoft Excel sheet. Peptide identity was ≥99%. Color codes show runs performed from tendons isolated at the same time. Data for fibronectin and periostin are highlighted in yellow.
- https://doi.org/10.7554/eLife.09345.024





