Mechanism of Na+-dependent citrate transport from the structure of an asymmetrical CitS dimer
Figures
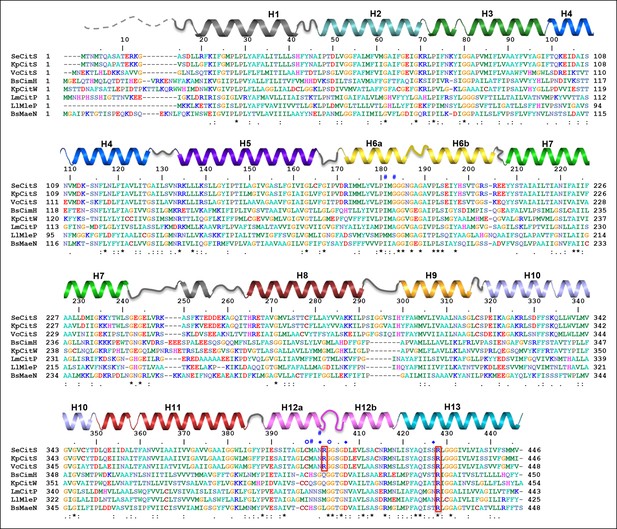
Sequence alignment.
Sequence alignment of 2-hydroxycarboxylate transporters. The secondary structure of SeCitS is shown above the alignment. R402 and R428 of the citrate-binding site are outlined in red. Symbols above the sequence indicate residues involved in sodium binding. A hashtag (#) marks the residues that form the Na1 site. Residues with sidechains coordinating Na2 are marked with a diamond (♦), and those that coordinate Na2 with backbone carbonyls with an open circle (○). Most of the conserved residues (*) are found in the two helix hairpins H6 and H12, and in transmembrane helix H13.
SeCitS: Citrate/sodium symporter from Salmonella Enterica (WP_024797394.1)
KpCitS: Citrate/sodium symporter from Klebsiella pneumoniae (WP_025860623.1)
VcCitS: Citrate/sodium symporter from Vibrio_cholerae (WP_001003397.1)
BsCimH: Citrate/malate transporter from Bacillus_subtillis, (P94363.1)
KpCitW: Citrate/acetate transporter from Klebsiella_pneumoniae, (AF411142.1)
LmCitP: Citrate transporter from Leuconostoc_mesenteroides (AAA60396.1)
LlMleP: Malate transporter from Lactococcus lactis, (CAA53590.1)
BsMaeN: Malate/sodium symporter from Bacillus_subtilis, (AFQ59004.1)
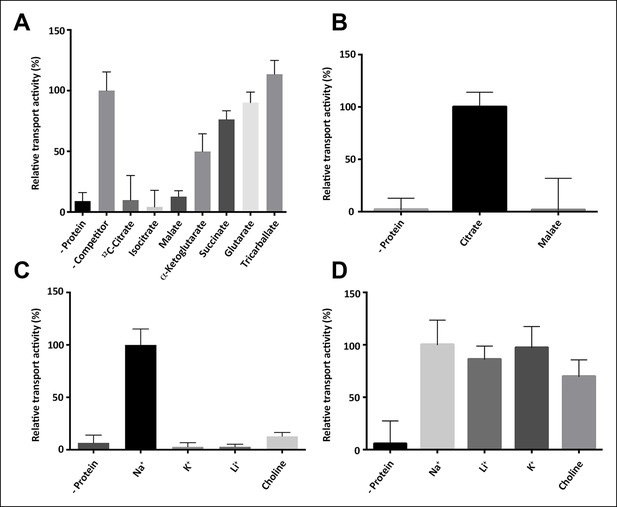
Substrate specificity of SeCitS.
(A) The substrate specificity of SeCitS was established by a proteoliposome uptake inhibition assay. Potential substrates or competitors were added in thousandfold excess of 14C-citrate (5 µM) and transport was measured. The 2-hydroxycarboxylates malate and iso-citrate inhibit 14C-citrate uptake completely. α-Ketoglutarate, which has a carbonyl instead of the citrate hydroxyl group, inhibits less strongly. Succinate and glutarate inhibit transport only slightly. Tricarballate has no effect. (B) While malate inhibits citrate uptake, it is not a substrate for SeCitS, as uptake of 14C-malate (43 µM) is not detectable. (C,D) SeCitS is highly specific for Na+. Neither Li+ nor K+ drive (C) or inhibit (D) citrate uptake. Choline was used as a negative control in both assays. Initial uptake rates were plotted relative to (A) absence of competitor, (B) citrate transport or (C,D) sodium-driven transport.
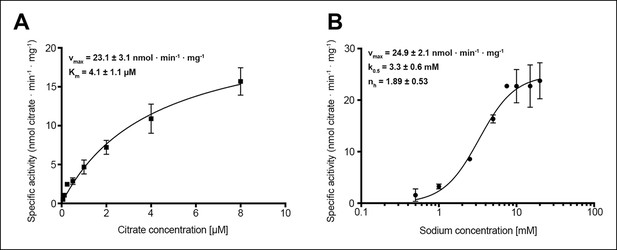
Citrate and sodium transport kinetics.
(A) Citrate uptake by SeCitS containing proteoliposomes in presence of 25 mM Na+ is non-cooperative and follows Michealis-Menten kinetics with a Km of 4.1 µM and a vmax of 23.1 nmol · min-1 · mg-1. (B) Na+ transport in presence of 5 µM citrate is cooperative, with a Hill coefficient of 1.89. The affinity of SeCitS for Na+ is lower than for citrate, as demonstrated by a Km of 3.3 mM. The vmax of 24.9 nmol · min-1 · mg-1 indicates a turnover rate of 1.2 citrate molecules per protomer per minute at room temperature.
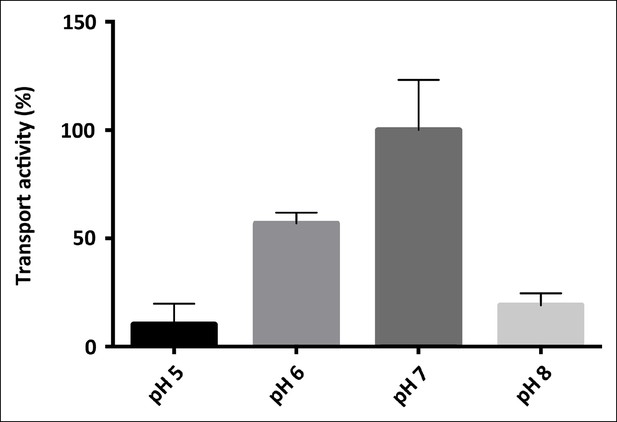
pH-dependence of SeCitS transport.
pH-dependent citrate uptake in proteoliposomes measured under symmetrical conditions (same pH inside and outside). SeCitS activity is maximal at pH7 (100%) and decreases to 55% at pH6. Down-regulation to 10% activity at pH5 or 20% at pH8 results in a roughly bell-shaped pH profile. Initial uptake rates were plotted relative to the activity maximum at pH7.
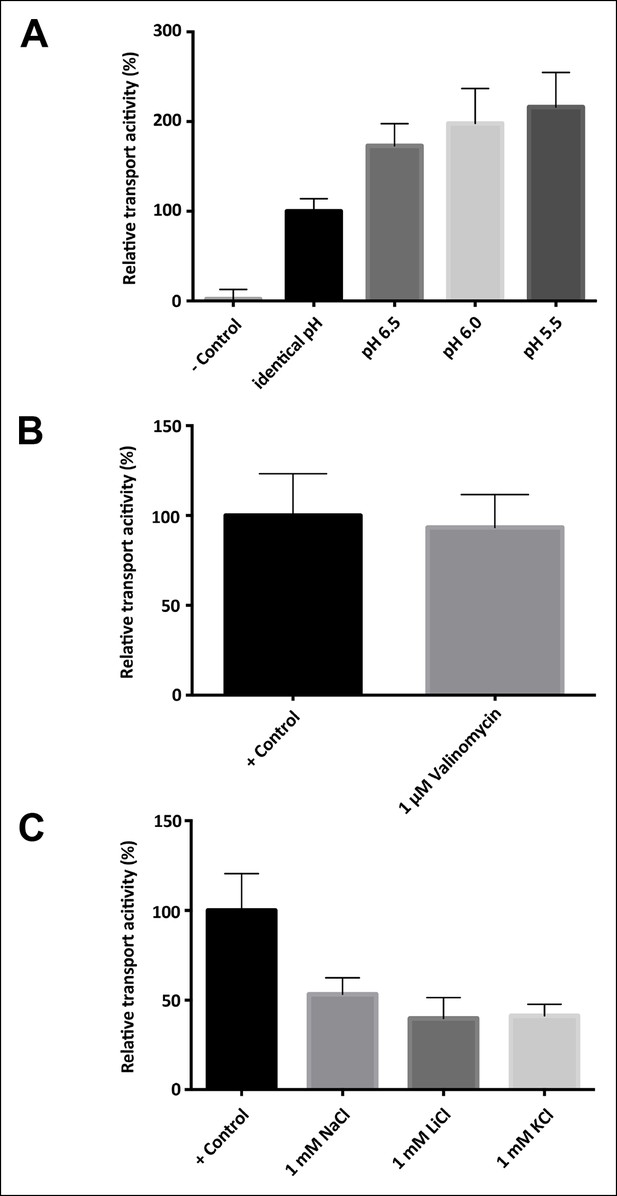
Driving force, electrogenicity and effect of internal salt concentration.
(A) Activity of SeCitS reconstituted into proteoliposomes with an inside pH of 7.0 and variable outside pH. A ΔpH increases the transport rate up to twofold compared to transport driven by sodium only. (B) Transport activity of SeCitS is electroneutral, as the addition of 1 µM valinomycin (pH 7.0, 5 mM KCl inside and outside) has no effect. (C) To investigate the influence of internal salt on the transport activity, SeCitS was reconstituted with either 1 mM Na+, Li+, K+, or choline. Transport is slightly inhibited by inside Na+, Li+, or K+. However, there is no difference between Na+, Li+, or K+, indicating that internal sodium does not favour transport. Transport rates were plotted relative to (A) transport under symmetrical pH, (B) transport without valinomycin or (C) transport in the absence of additional, intraliposomal Na+, K+ or Li+.
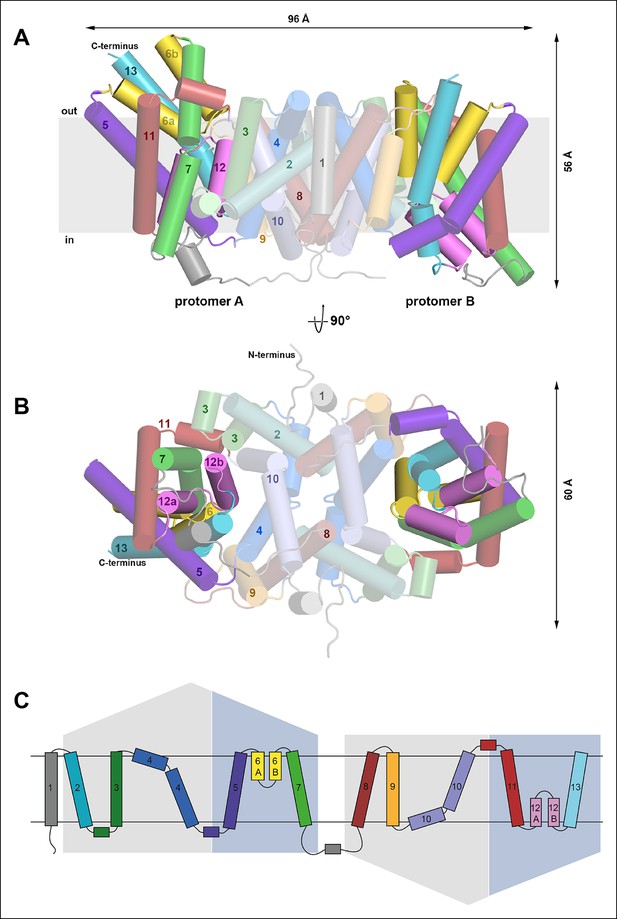
Overall structure of SeCitS and topology.
Side view (A) and cytoplasmic view (B) of the SeCitS homodimer. The dimer is oval, with a long axis of 96 Å and a short axis of 60 Å. Each protomer consists of eleven transmembrane helices and two helix hairpins (yellow and pink). (C) SeCitS consists of two inverted 5-TMH repeats connected by a long cytoplasmic loop plus an additional N-terminal helix. Each repeat contains one hairpin. Helices belonging to the helix bundle are shown on blue background, while helices of the dimer contact domain are shown on grey background. The extended flexible link between the two inverted repeats is completely resolved in protomer A (A).
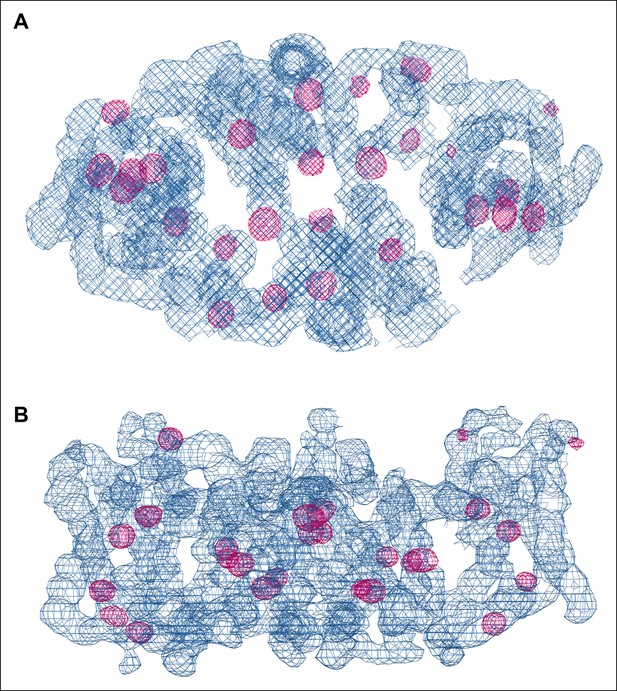
SeMet phasing.
Top view (A) and side view (B) of electron density (blue, 1.5 σ) of one dimer in the asymmetric unit after phasing and density modification. Strong Se peaks in the anomalous difference map contoured at 5 σ (magenta) indicate SeMet positions. Out of 72 potential selenium sites in the asymmetric unit, 53 were found in the substructure with an occupancy > 20%.
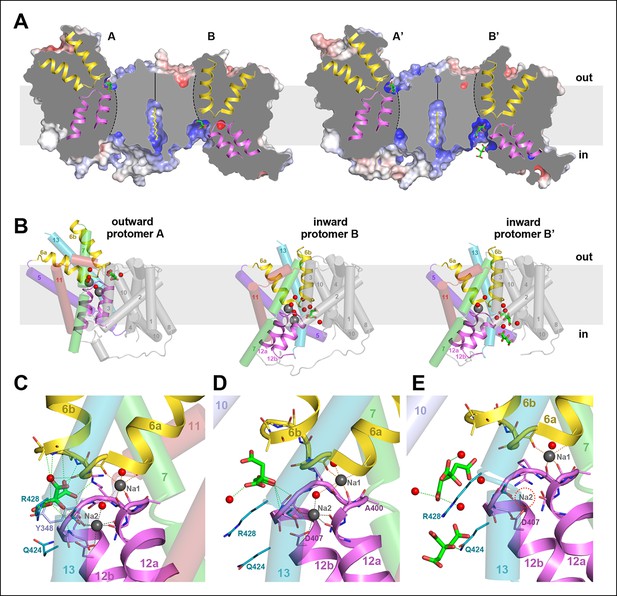
Two different states of the asymmetrical SeCitS dimer.
(A) The outward-facing protomers A and A' bind citrate in a shallow, positively charged cavity between the helix bundle and dimer contact domain. In the inward-facing protomers B and B', citrate binds in a deep cytoplasmic cavity. In B', two citrate molecules are resolved. (B) In protomers A, A’ and B, two Na+are occluded in the helix bundle, while in B' only one Na+ is present. The substrates are translocated 16 Å across the membrane by a 31° rotation of the helix bundle relative to the static dimer contact domain. (C) In the outward-facing protomers, citrate is closely coordinated by sidechains of both hairpins and H13. Neither Na+ participates directly in citrate coordination. (D) In the inward-facing protomer B, citrate is hydrated and attached weakly to the glycine-rich loop of H12. The Na1 and Na2 sites in (C) and (D) are virtually identical, indicating that the transition from the outward-facing to the inward-facing state does not affect Na+-coordination geometry. (E) In protomer B', only the Na1 site is occupied. Two citrate molecules are resolved, outlining a likely trajectory for citrate release (Video 1).
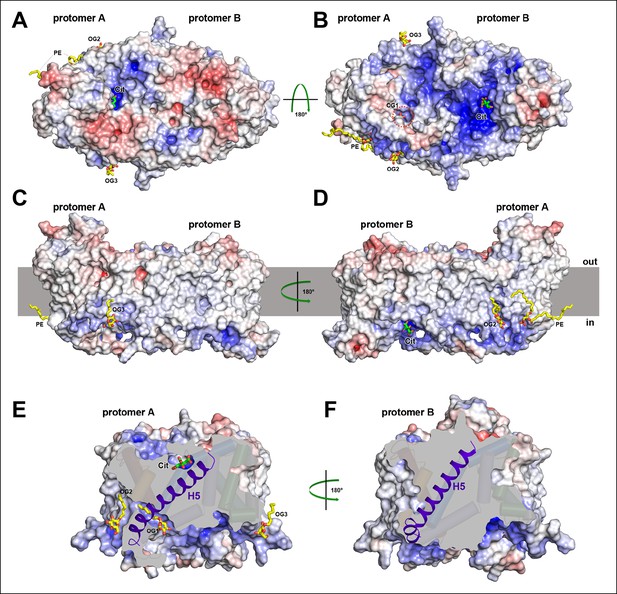
Electrostatic surface potential and bound detergent/lipid molecules.
Exterior (A) and cytoplasmic views (B) of the electrostatic surface potential of SeCitS accentuates the dimer asymmetry. The binding sites for the citrate di-anion (green) on the exterior surface of protomer A and the cytoplasmic side of protomer B are strongly positively charged (dark blue). (C, D) Positions of bound detergent and lipid molecules (yellow) are shown in the side view of the electrostatic surface. Apart from the aliphatic chain in the hydrophobic cavity of the dimer interface (Figure 5), they are positioned close to the helix bundle. (E) In the outward-facing protomers, a hydrophobic cavity between H5, H13 and the dimer contact domain is filled by a detergent molecule. This cavity is closed in the inward-facing protomers (F).
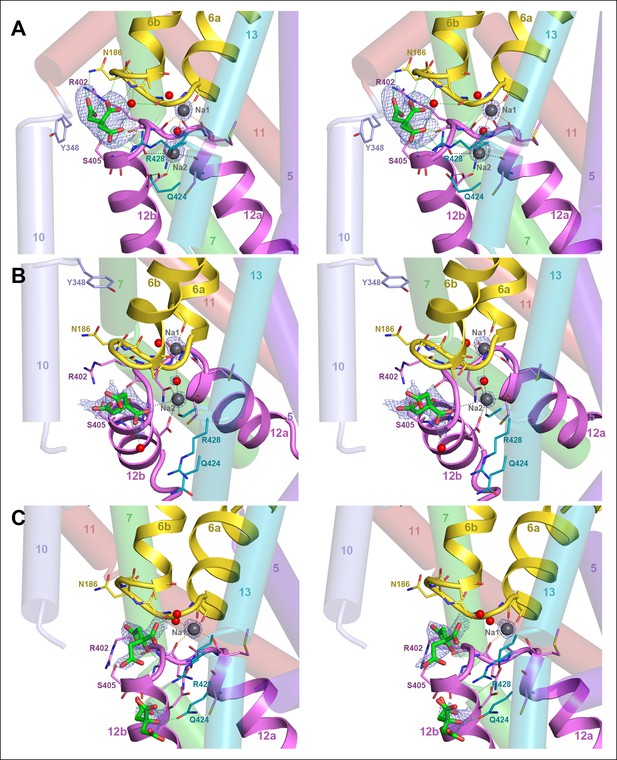
Binding sites and Fo-Fc ligand density.
(A) Stereo view of the outward-facing substrate-binding site of protomer A with an extensively coordinated citrate molecule. (B) In the inward-facing binding site of protomer B the citrate is attached less strongly. In (A) and (B) the Fo-Fc density (blue mesh) is contoured at 3σ for citrate and at 5σ for the two bound Na+ ions and the water molecule between them. (C) In the inward-facing protomer B’, the Fo-Fc map contoured at 4σ shows an occupied Na1 site, while the Na2 site is empty. The Fo-Fc omit map contoured at 2.5 clearly shows two citrate molecules.
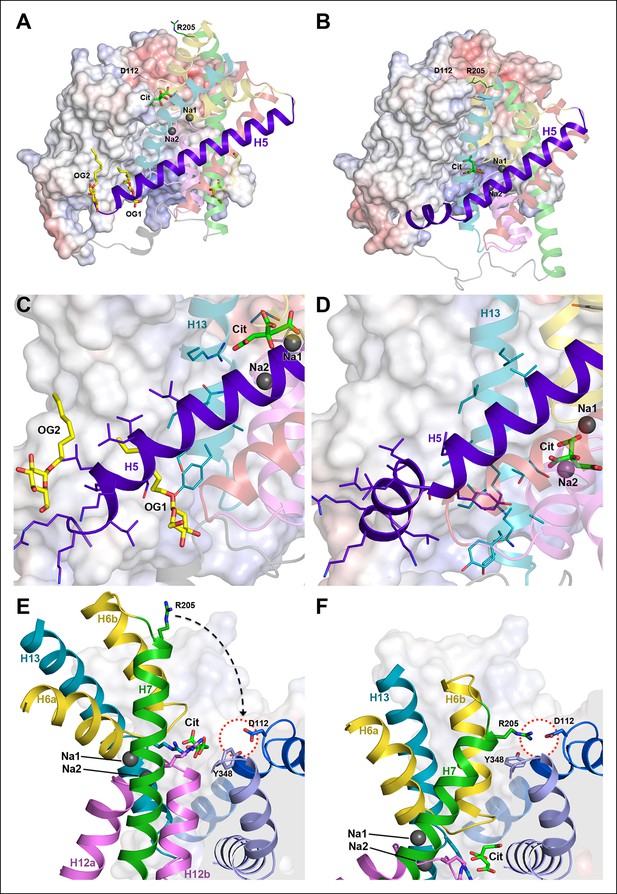
Hydrophobic interface between helix bundle and dimer contact domain.
(A, C) In the outward-facing protomers A and A’, a hydrophobic pocket between helix H5, H13 and the dimer contact domain harbors a detergent molecule that apparently replaces a membrane lipid. (B, D) In the inward-facing protomers B and B' H5 kinks at Gly143 and shifts towards the cytoplasm. We assume that H13 fills this hydrophobic cavity in the inward-facing state. (E) In the outward-facing protomers, Tyr348 coordinates the citrate by π-π-interactions. As a result of the arc-like helix bundle rotation, an ion bridge forms between Asp112 and Arg205 (H7) in the inward-facing protomers (F). Arg205 moves by more than 20 Å from its position in the outward-facing conformation (E). The sidechain of Tyr348 rotates by 90°, blocking the entrance to the substrate binding site (F).
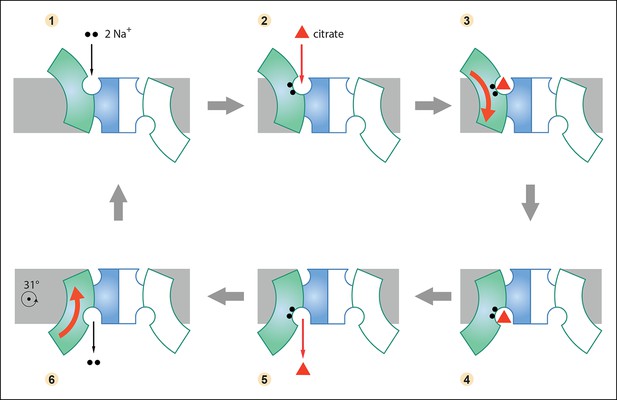
Six-step mechanism of Na+-dependent citrate uptake by SeCitS.
(1) Two Na+ bind to the empty transporter; (2) citrate from the external medium attaches to the binding site; (3) the substrates are translocated across the membrane through a rigid-body 31° rotation of the helix bundle domain; (4) first the citrate and then (5) the Na+ ions are released to the cytoplasm; (6) the unloaded protomer changes its conformation back to the outward-facing state and the cycle restarts. In the cell, the inward-directed Na+gradient drives citrate uptake, but all steps are in principle reversible. The approximate position of the rotation axis parallel to the membrane and perpendicular to the long dimer axis is indicated in (6).
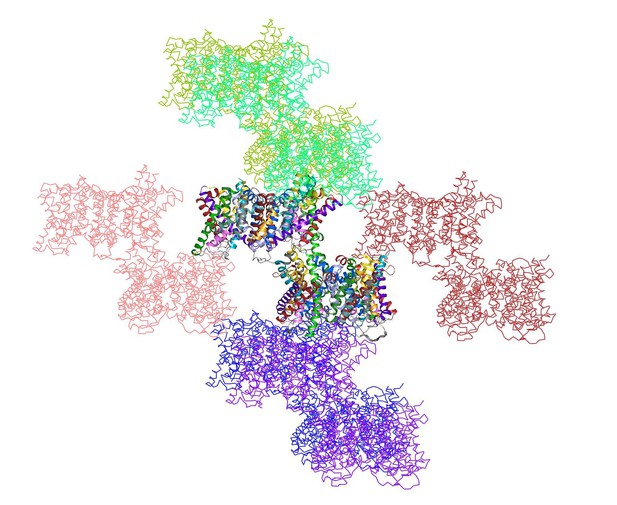
shows the two dimers in the asymmetric unit (multi-coloured) surrounded by 12 symmetry-related dimers.
https://doi.org/10.7554/eLife.09375.018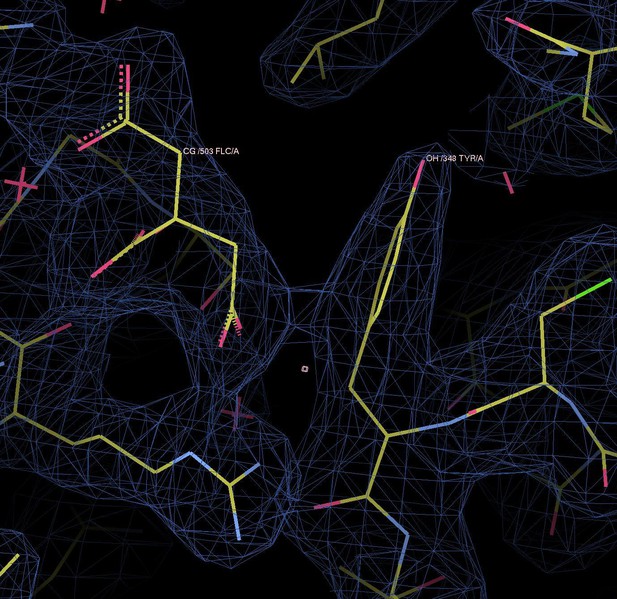
Videos
Schematic representation of SeCitS transport.
The movie shows a morph from the outward-facing to the inward-facing conformation for one protomer of the SeCitS dimer. Arg402, Arg428 and Tyr348, which coordinate citrate in the outward-facing conformation, are drawn as stick models, while the Na+ ions are represented as grey spheres. Na+ ions bind to their respective sites in the helix bundle, followed by citrate binding between helix bundle and dimer contact domain. Subsequently, the substrates are translocated by a rotation of the bundle. Citrate release is independent from the release of either Na+ ion. Due to the empty Na2 binding site in protomer B’ we assume that this ion is released immediately after the citrate. After substrate release the empty transporter changes its conformation back to the outward-facing state to repeat the cycle.
Schematic representation of domain, helix and sidechain movements.
Three synchronized movies show different views of one SeCitS protomer during the transport cycle: (A) from the membrane plane, (B) in the perpendicular direction from the cell exterior and (C) a detailed view of the substrate-binding site and the detergent/lipid binding pocket. Helices of the rotating bundle domain are coloured, while helices in the static dimer contact domain are shown in grey behind their corresponding transparent electrostatic surface. The negatively charged periplasmic surface of SeCitS (transparent red) attracts Arg205 of H7 (green), which, in the inward-facing state, forms an ion bridge to Asp112 in H4 and a hydrogen bond to Tyr348 (lavender) in H10 of the dimer contact domain. In the outward-facing state, Asp112 interacts with Tyr348, which rotates to block access to the substrate-binding site in the inward-facing state. A detergent molecule (yellow) in the hydrophobic pocket between H5 (purple), H13 (cyan) and the dimer contact domain, is displaced in the inward-facing state by the movement of H13. H5, which is straight in the outward-facing state, kinks during the bundle rotation to prevent its partial exposure to the cytoplasm.
Tables
Data collection and refinement statistics
| Native SeCitS | SeMet SeCitS | |
|---|---|---|
| Data collection | SLS PXII | |
| Wavelength (Å) | 0.979 | 0.980 |
| Space group | P1 | P21 |
| Cell dimensions | ||
| a, b, c (Å) | 86.4, 89.9, 91.8 | 90.9, 168.8, 97.9 |
| α, β, γ (°) | 90.4, 113.8, 99.5 | 90.0, 91.0, 90.0 |
| Resolution (Å) | 47.98 – 2.5 (2.6 – 2.5) | 48.95 – 3.9 (4.0 -– 3.9) |
| Rpim | 0.052 (0.872) | 0.038 (0.539) |
| I / σI | 8.9 (1.3) | 16.8 (2.2) |
| CC* | 0.999 (0.828) | 1.000 (0.944) |
| Completeness (%) | 98.8 (98.1) | 100 (100) |
| Multiplicity | 8.2 (8.1) | 41.4 (40.9) |
| Refinement | ||
| Resolution (Å) | 47.98 – 2.5 (2.6 – 2.5) | |
| Unique reflections | 84765 | |
| Reflections in test set | 4193 | |
| Rwork/Rfree (%) | 21.0/24.8 (33.6/36.3) | |
| CC(work)/CC(free) | 0.848/0.742 (0.796/0.773) | |
| Average B-Factor (Å2) | 70 | |
| No. atoms in AU | 13270 | |
| Protein | 12916 | |
| Ligands | 285 | |
| Water | 69 | |
| r.m.s. deviations: | ||
| Bond lengths (Å) | 0.003 | |
| Bond angles (°) | 0.762 | |
-
Values for the highest resolution shell are shown in parentheses





