Claudin-2-dependent paracellular channels are dynamically gated
Figures
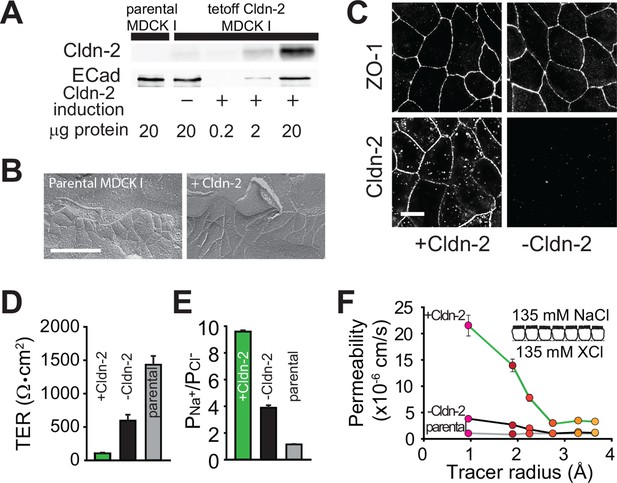
Claudin-2 expression enhances tight junction permeability to small cations.
(A) Transgenic MDCKI monolayers were developed to express claudin-2 (+Cldn-2) in the absence of doxycycline. Limited claudin-2 expression was detected in the absence of induction and none was detectable in the parental MDCKI line. (B) Induction of claudin-2 expression had no effect on tight junction ultrastructure (Bar = 500 nm). (C) Tight junction claudin-2 was not detectible by immunofluorescence staining after suppression of claudin-2 expression (Bar = 10 µm). (D) Claudin-2 expression reduced TER (E) and increased relative permeability of sodium to chloride (PNa+/PCl-) was increased. (F) Biionic potential analyses show that the reduction in TER was mainly due to increased paracellular permeability to small cations.
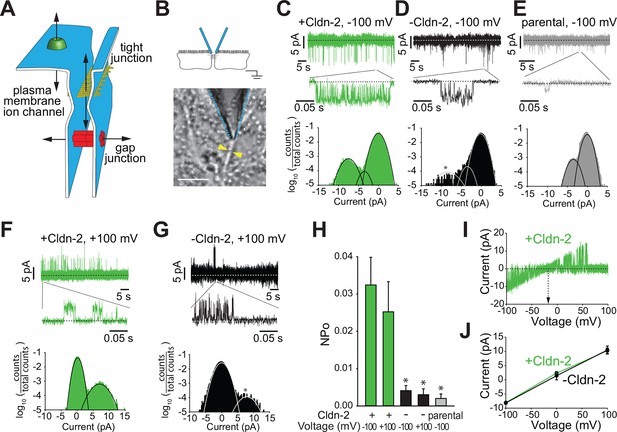
Claudin-2 expression correlates with the frequency of local tight junction channel openings in MDCKI monolayers.
(A) Tight junctions are distinct from plasma membrane ion channels and differ from gap junctions in their ability to define conductance between two extracellular compartments. (B) Trans-tight junction patch clamp placement. Yellow arrowheads show intercellular junction (Bar = 10 μm). (C) Conductance events detected at −100 mV when claudin-2 was expressed (+Cldn-2). (D) In the absence of induced claudin-2 expression (–Cldn-2), the frequency of similar sized conductance events was dramatically reduced. (E) Small claudin-2 independent events were present in parental MDCKI monolayers (F) Conductance events were present at +100 mV when claudin-2 was expressed (+Cldn-2). (G) Events were infrequent in the absence of induced claudin-2 expression (–Cldn-2). (H) NPo was reduced by 87% ± 4% (at –100 mV) and 88% ± 6% (at +100 mV) after suppression of claudin-2 expression. Events were rare in recordings from parental tight junctions. (I) Representative recording of voltage ramp in claudin-2-expresing MDCKI monolayers showing linear current voltage relationship and reversal potential close to 0 mV. (J) Average current voltage relationships (n = 8 to 32 per condition) reveals that average channel conductance was ~90 pS regardless of whether claudin-2 expression was induced (green line) or not (black line).
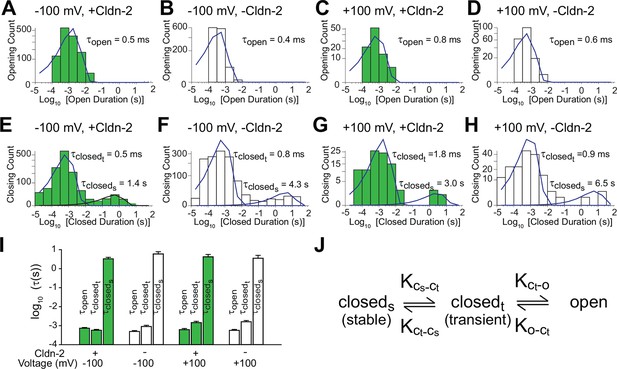
Patch clamp recordings reveal a single open state and two closed states.
(A–D) A single population of fast openings was observed in the presence (green) or absence (white) of induced claudin-2 expression at –100 and +100 mV (a-d total recording times: 40 s, 225 s, 31 s, 52 s.). (E–H) Corresponding closed duration histograms from the same representative recordings reveal two distinct closed states. (I) Opening and closing time constants were voltage independent and were similar with and without claudin-2 induction (n=7 to 35 recordings for each condition). (J) Kinetic analysis demonstrates the presence of both stable (cstable) and transient (ctransient) closed states and one and open (o) state.
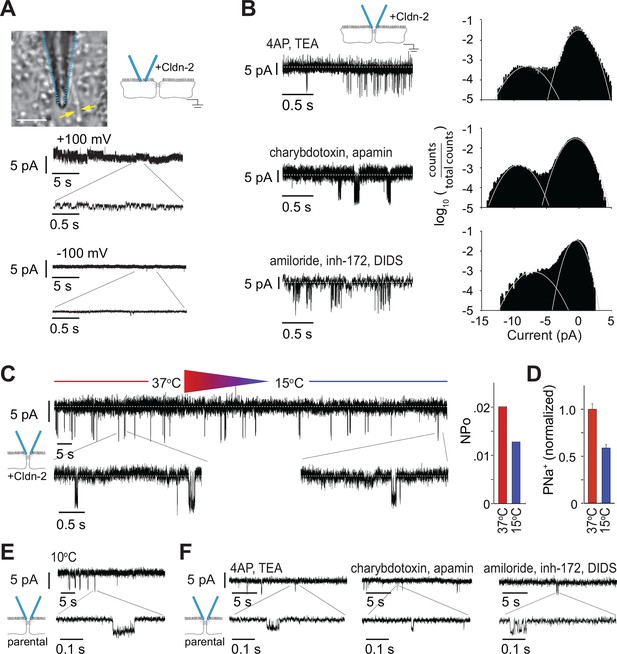
Large and small tight junction currents are not due to transmembrane ion channels.
(A) Small (<2 pA) transmembrane ion channel openings were detectable in off-tight junction recordings after applying a 500 Hz low pass filter. (Bar = 10 μm). (B) Events detected by trans-tight junction patch clamp were not blocked by three different ion channel inhibitor cocktails (+Cldn-2; representative of n = 3 to 8 per condition). (C) Monolayers were cooled while recording from trans-tight junction patch clamp. The number of events detected was reduced at 15°C, relative to 37°C, but event amplitude was unaffected (+Cldn-2; representative of n = 4). (D) NPo and Na+ permeability measured across a 0.33 cm2 monolayer were similarly reduced at 15°C (+Cldn-2). (E) ~4 pA events remained detectable after chilling monolayers to 10°C (MDCKI parental monolayers; representative of n = 4). (F) ~4 pA events were not blocked by three different ion channel inhibitor cocktails (MDCKI parental monolayers; representative of n = 3 to 5 per condition).
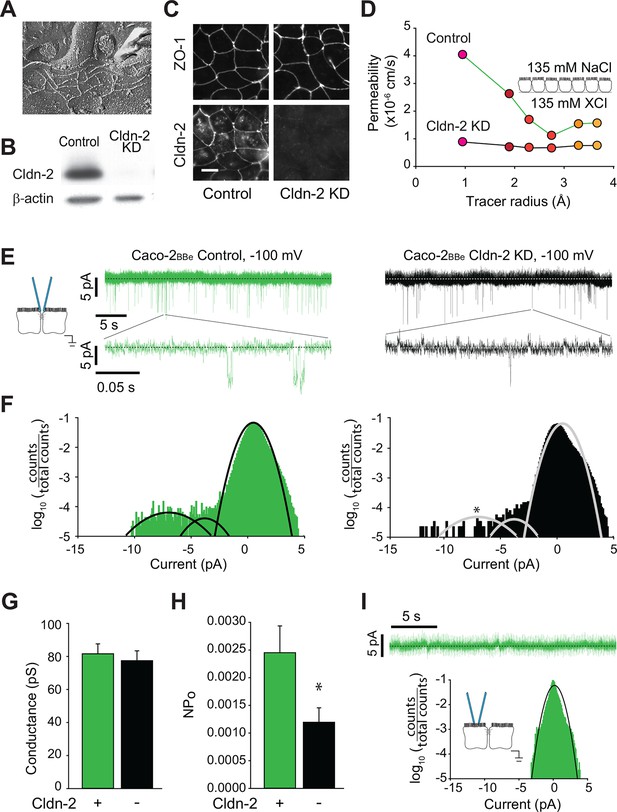
Global conductance and trans-tight junction patch clamp event frequency correlate with claudin-2 expression in Caco-2BBe intestinal epithelial monolayers.
(A) Freeze fracture electron microscopy demonstrating that mature tight junctions in Caco-2BBe monolayers are composed of 3–5 strands (Shen et al., 2006), similar to MDCKI. (B) Western blot confirms >99% knockdown of claudin-2 in Caco-2BBe monolayers. (C) Claudin-2 is not detectable by immunofluorescence microscopy of knockdown Caco-2BBe monolayers (Bar = 10 µm). (D) Biionic potential analyses show that claudin-2 knockdown reduces small cation permeability. (E) Trans-tight junction patch clamp recordings of Caco-2BBe cells detected events at −100 mV (n=5 per condition). Representative traces of trans-tight junction patch clamp data from control and claudin-2 knockdown Caco-2BBe monolayers. (F) All points histogram analysis of patch clamp data from Caco-2BBe monolayers shows a specific reduction in ~9 pA events with no change in frequency of ~4 pA events after claudin-2 knockdown. (G) Average opening conductances was unaffected by the levels of claudin-2 expression. (H) Channel activity (NPo) was reduced by claudin-2 knockdown (n = 5 to 7 per condition). (I) Neither ~9 pA nor ~4 pA events were not detectable when the pipette was sealed away from the tight junction in Caco-2BBe monolayers (n = 12).
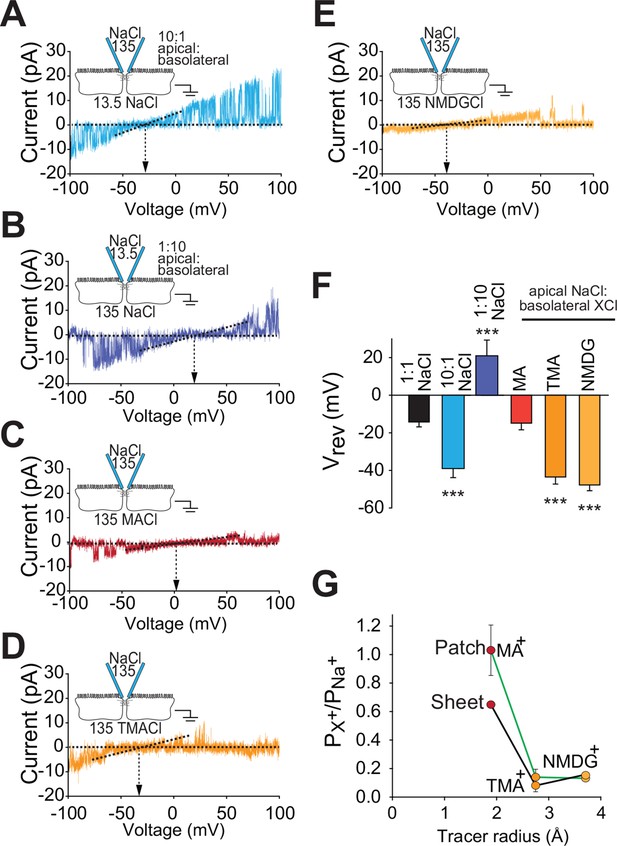
Claudin-2-dependent conductance events measured by trans-tight junction patch clamp display cation- and size-selective properties similar to transepithelial paracellular conductance measured over large areas.
(A–E) Current-voltage (I-V) relationships for events detected by trans-tight junction patch clamp in MDCKI monolayers with transgenic claudin-2 expression (+Cldn-2) under the ionic conditions shown. Pipette and basolateral buffer composition are indicated in mM. (F) Vrev was determined under each of the ionic conditions shown (n=4 to 7 per condition). (G) Cation permeability determined from shifts in trans-tight junction patch clamp Vrev (green line) or traditional (black line) bi-ionic potential measurements.
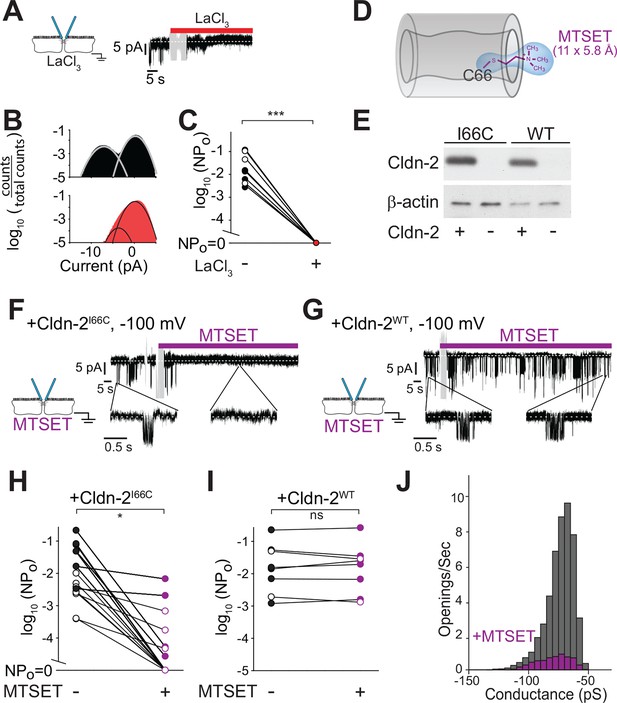
Paracellular conductance events are blocked by La3+ or claudin-2 derivatization.
(A) LaCl3 (red bar) blocked opening events. Solution exchange artifact is shown in gray. (B) The ~9 pA events were eliminated from the all-points histogram by La3+ treatment (before LaCl3 black; after LaCl3 red) (C) La3+ treatment (red) reduced NPo to 0 (closed symbols indicate measurements at -100 mV, open symbols indicate measurements at +100 mV, n = 9). (D) MTSET forms a disulfide bond with Cys66 located within the pore of claudin-2I66C channels (Angelow and Yu, 2009). (E) Transgenic claudin-2I66C was expressed at levels similar to claudin-2WT. (F,G) MTSET dramatically reduced the number of detectable events in trans-tight junction patch clamp recordings from MDCKI cells expressing claudin-2I66C within ~20 s, but had no effect on monolayers expressing claudin-2WT. Blue bar indicates presence of MTSET (n = 8 to 16 per condition). Solution exchange artifacts are shown in gray. (H,I) NPo of MDCKI cells expressing claudin-2I66C or claudin-2WT before and after (purple) MTSET treatment (closed symbols indicate measurements at -100 mV, open symbols indicate measurements at +100 mV, n = 8 to 16 per condition). (J) Derivatization of claudin-2I66C does to affect conductance of residual events (V = –100 mV). The histogram depicts frequency of events before and after (purple) MTSET treatment.
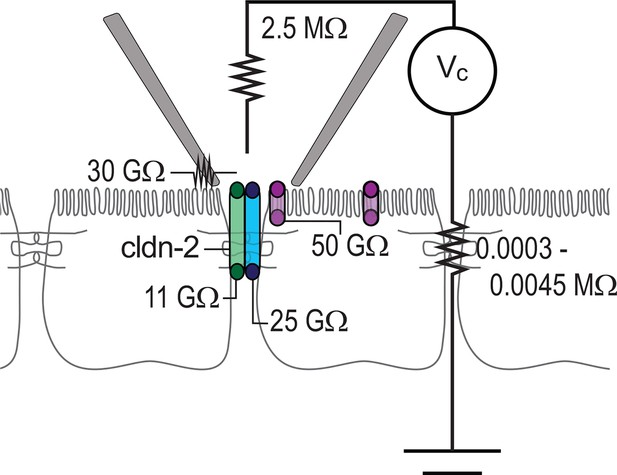
Circuit analysis of current pathways detected by trans-tight junction patch clamp.
Resistances of the pipette seal, the electrode, the paracellular pathway of the larger epithelial sheet (outside of the patch), detectable apical membrane channels, and both claudin-2-dependent (green) and claudin-2-independent (blue) paracellular channels are shown. Both paracellular channels were detected only when the patch pipette was sealed over the intercellular junction. The apical membrane channels were only detected when the patch pipette was sealed to away from the intercellular junction, but, are likely present within the apical membrane adjacent to the junction as well.





