Fcp1 phosphatase controls Greatwall kinase to promote PP2A-B55 activation and mitotic progression
Figures
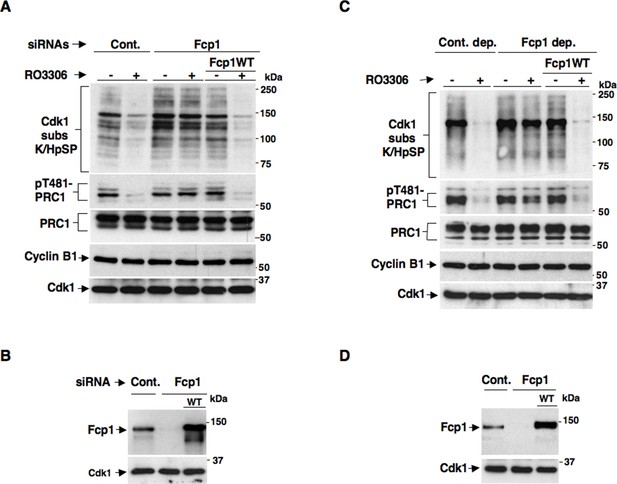
Fcp1 affects PP2A-B55-dependent dephosphorylations at mitosis exit.
(A, B) Control (Cont.) or Fcp1-depleted (Fcp1) by siRNAs, as well as Fcp1-depleted complemented with wild type Fcp1 (Fcp1WT), HeLa cells were arrested at prometaphase. (A) Cells were collected and split into two samples, one sample received vehicle (-), the other RO3306 (+), and they were further incubated for 15 min, lysed and lysates probed for indicated antigens. (B) Cells were lysed and lysates probed for indicated antigens. (C, D) Mitotic HeLa cell extracts were mock-depleted (Cont. dep.), Fcp1-depleted (Fcp1 dep.) or Fcp1-depleted and reconsituted with Fcp1WT. (C) Each set was split into two portions, one received just vehicle (-), the other RO3306 (+), and they were incubated for 30 min at 23°C and probed for indicated antigens. (D) Before incubation, extracts samples were also probed for Fcp1 and Cdk1. The data shown are representative of three independent experiments per type.
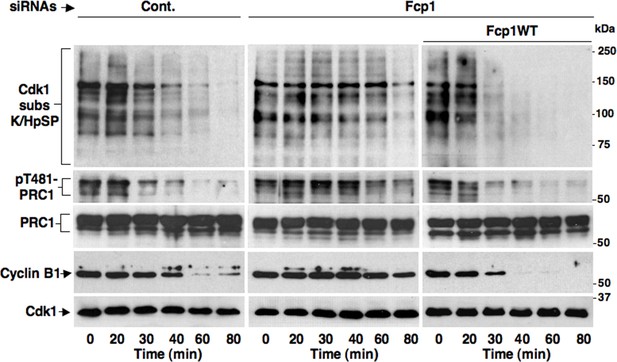
Fcp1-dependency of mitotic exit dephosphorylations.
Control (Cont.) or Fcp1-depleted (Fcp1), as well as Fcp1-depleted complemented with wild type Fcp1 (Fcp1WT), HeLa cells were arrested at prometaphase. Lysates from cells released from prometaphase arrest and sampled at indicated time points of incubation were probed for indicated antigens. Fcp1-depletion efficiency is depicted in Figure 1B. The data shown are representative of three independent experiments per type.
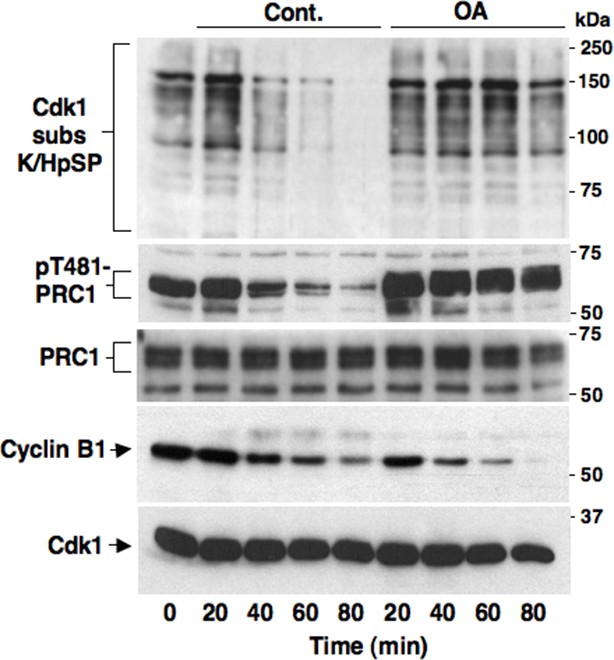
Okadaic acid-sensitive mitotic exit dephosphorylations.
Prometaphase-arrested HeLa cells were collected and, immediately after taking the time 0 sample, split into two portions, one portion received vehicle (Cont.) and the other 500 nM okadaic acid (OA); then, samples were taken at indicated time points of further incubation. Cell lysates were separated on SDS/PAGE and probed for indicated antigens. Data shown are representative of at least three independent experiments.
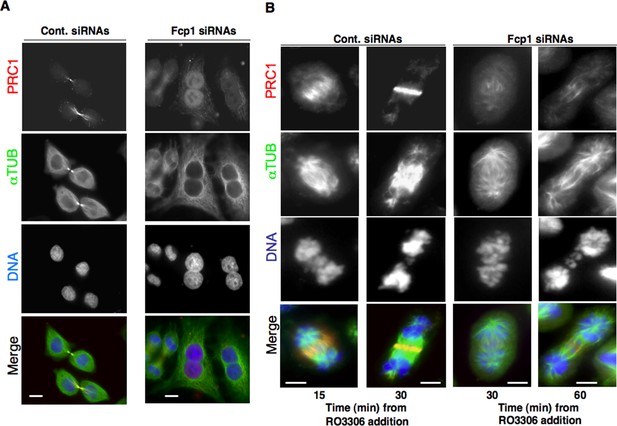
PRC1 localization in Fcp1-depleted cells.
(A) Asynchronously growing control (Cont. siRNAs) or Fcp1-siRNAs-treated (Fcp1 siRNAs) HeLa cells were fixed and stained for PRC1 (red), α-tubulin (green) and DNA (blue). (B) Control (Cont. siRNAs) or Fcp1-siRNAs-treated (Fcp1 siRNAs) HeLa cells, previously arrested at prometaphase, were released into fresh medium in the presence of the proteasome inhibitor MG132. After 30 min incubation, the Cdk1 inhibitor RO3306 was added, cells were fixed and stained for PRC1 (red), α-tubulin (green) and DNA (blue) at indicated time points of further incubation. Scale bars, 10 μm. Data shown are representative of three independent experiments.
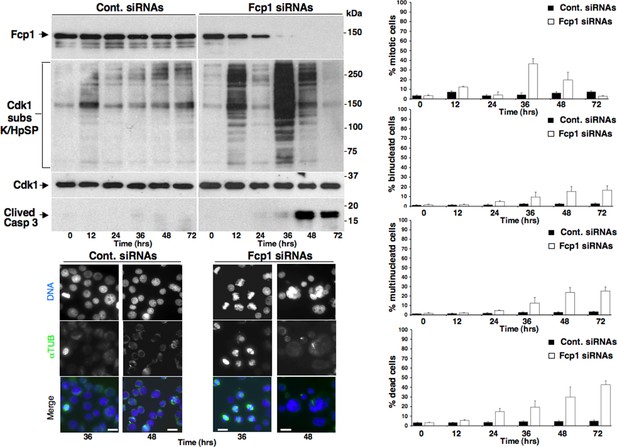
Mitotic phenotypes in Fcp1-depleted cells.
Asynchronously growing control-siRNAs- (Cont. siRNAs) or Fcp1-siRNAs-treated (Fcp1 siRNAs) HeLa cells were taken at the indicated time points (hours, hrs) from the siRNAs treatment. Upper left panels, cell lysates were separated on SDS/PAGE and probed for indicated antigens including clived caspase 3 (Clived Casp 3). Lower left panels, cells were fixed and stained for DNA (blue) and α-tubulin (green). Rigth graphs, percentage of mitotic, binucleated and multinucleated cells, scored visually from three fields per sample after staining as described for the lower left panels (about 100 cells per field; cells were scored as mitotic from late prophase to anaphase-telophase) and of death cells, scored visually by trypan blue inclusion from triplicates per sample. Scale bars, 10 μm. Data shown are representative of three independent experiments.
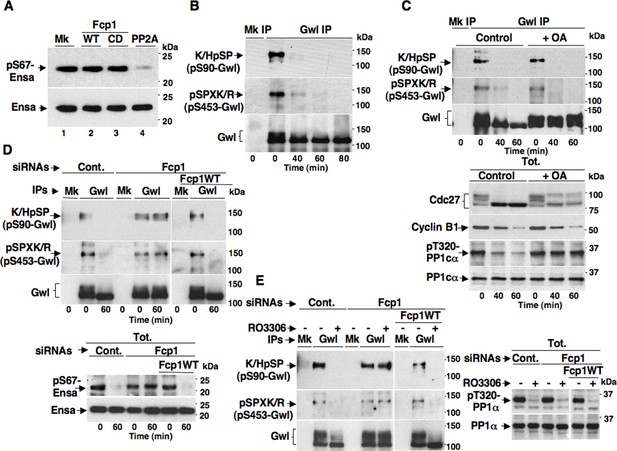
Fcp1-dependency of Gwl dephosphorylation at S90 and S453 during mitosis exit.
(A) Ensa IP from prometaphase-arrested HeLa cells, previously transfected with a Flag-hEnsa expression vector, was divided into four sets and incubated as substrate in phosphatase reactions with either just buffer (Mk) or with purified active Fcp1 (WT), or an inactive, catalytic dead, Fcp1 version (CD), or active PP2A for 60 min at 30°C (lanes 1, 2, 3 and 4, respectively). The reactions were then probed for pS67Ensa and Ensa content (the Fcp1WT and Fcp1CD protein preparations were the same used in the experiment depicted in Figure 3C; see below). (B) Prometaphase-arrested HeLa cells were released into fresh medium and sampled at indicated time points of further incubation, lysed and processed for mock (Mk) or Gwl IP. IPs were separated in parallel SDS/PAGE and probed for indicated antigens. (C) Prometaphase-arrested HeLa cells were released into fresh medium and split into two portions; then, immediately after taking the time 0 sample, vehicle (Control) or 2 μM okadaic acid (+ OA) were added. Cells were further sampled at indicated time points of incubation. Upper panels, cell lysates were processed for mock (Mk) or Gwl IP that were probed for indicated antigens; lower panels, total cell lysate samples (Tot.) were probed for indicated antigens. (D) Control (Cont.) or Fcp1-depleted (Fcp1), as well as Fcp1-depleted complemented with wild type Fcp1 (Fcp1WT), HeLa cells were arrested at prometaphase, released and sampled at the indicated time points of incubation. Upper panels, cell lysates were processed for mock (Mk) or Gwl IP that were probed for indicated antigens; lower panels, total cell lysate samples (Tot.) were probed for indicated antigens. (E) Control (Cont.) or Fcp1-depleted (Fcp1), as well as Fcp1-depleted complemented with wild type Fcp1 (Fcp1WT), HeLa cells were arrested at prometaphase and split into two samples, one sample received vehicle (-) the other RO3306 (+), further incubated for 15 min. Left panels, cell lysates were processed for mock (Mk) or Gwl IP that were probed for indicated antigens; right panels, total cell lysate samples (Tot.) were probed for indicated antigens. The levels of Fcp1 depletion and complementation were similar to those shown in Figure 1B. Data shown are representative of at least four independent experiments per type.
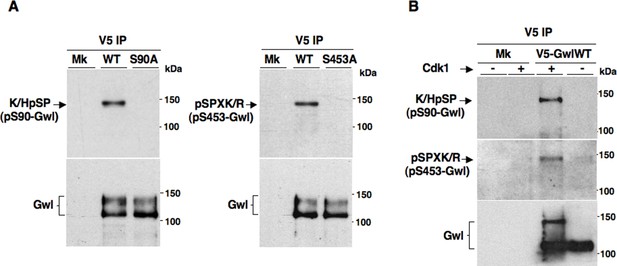
Cdk1-dependent phosphorylations of Gwl.
(A) Mock- (Mk), V5-GwlWT-, V5-GwlS90A- and V5-GwlS453A-transfected HeLa cells were arrested at prometaphase, lysed and processed for V5 IP. IPs were separated in parallel SDS/PAGE and probed for indicated antigens. (B). Mock- (Mk) and V5-GwlWT-transfected HeLa cells were arrested at prometaphase and released. Cells were taken after 120 min of incubation, to allow complete transition into the G1 cell cycle phase, and lysates processed for V5 IP. IPs were split into two portions and incubated in kinase reactions at 37°C for 20 min – or + active Cdk1 (Cyclin A-Cdk1, 126 ng per reaction), before being separated in parallel SDS/PAGE and probed for indicated antigens. Data shown are representative of at least four independent experiments per type.

Effect of prolonged Cdk1 inhibition on Gwl dephosphorylation in Fcp1-depleted cells.
Control (Cont.) or Fcp1-depleted (Fcp1) HeLa cells were arrested at prometaphase, split into two samples, one sample receiving vehicle (-), the other RO3306 (+), and further incubated for 30 min. Cell lysates were processed for mock (Mk) or Gwl IP that were then probed for indicated antigens. Data shown are representative of at least four independent experiments per type.
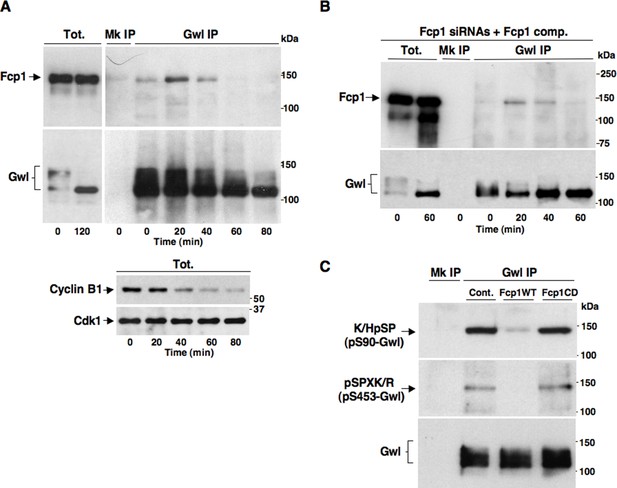
Fcp1 binds and dephosphorylates Gwl during mitosis exit.
(A) Prometaphase-arrested HeLa cells were released into fresh medium and sampled at indicated time points of incubation. Upper panels, cell lysates were processed for mock (Mk) or Gwl IP that were probed for indicated antigens; lower panels, total cell lysate samples (Tot.) were probed for indicated antigens. (B) Prometaphase-arrested, Fcp1-siRNAs-transfected and complemented with siRNAs-resistant Fcp1WT (Fcp1-siRNAs +Fcp1 comp.) HeLa cells were released into fresh medium and sampled at indicated time points of incubation. Total lysates (Tot.), mock (Mk) or Gwl IPs were probed for indicated antigens. Data shown are representative of at least three independent experiments per type. (C) Gwl IP from prometaphase-arrested HeLa cell lysates was divided into three sets and incubated in phosphatase reactions with either just buffer (Cont.), Fcp1WT or Fcp1CD proteins (Mk IP; 1/3 mock IP incubated with buffer) for 60 min at 30°C. Then, IPs were washed and probed for indicated antigens. Data shown are representative of three independent experiments per type.

Fcp1-Gwl interaction in hTERT-RPE1 cells.
hTERT-RPE1 cells were arrested at prometaphase, collected, released from arrest and sampled at the indicated time points during further incubation in fresh medium. Mock (Mk) or Gwl immunoprecipitates (IPs) from cell lysates and total cell lysate (Tot.) samples were separated in parallel SDS/PAGE and probed for indicated antigens. The data shown are representative of two independent experiments.
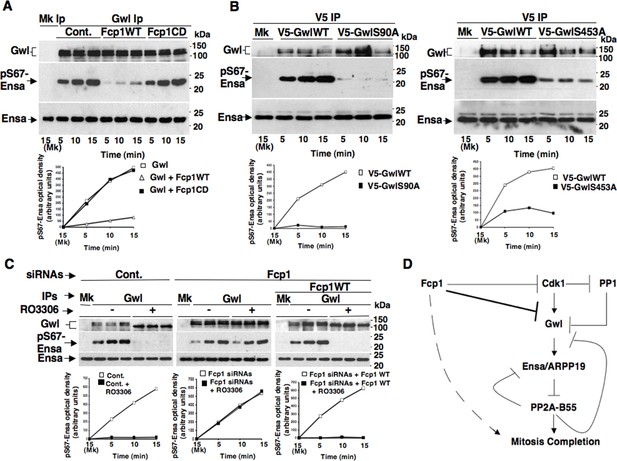
Fcp1-dependent dephosphorylation reduces Gwl kinase activity towards Ensa.
(A) Gwl IP from prometaphase-arrested HeLa cell lysates were divided into three sets and incubated for phosphatase reactions with either just buffer (Cont.), Fcp1WT or Fcp1CD proteins (Mk IP; 1/3 mock IP incubated with buffer) for 60 min at 30°C. Then, each IP set was split into three portions and incubated in kinase reactions with recombinant Ensa protein. (B) V5 IP from lysates of prometaphase-arrested HeLa cells, previously transfected with V5-GwlWT and V5-GwlS90A or V5-GwlS453A, were split into three portions and incubated at 37°C in kinase reactions with recombinant Ensa protein. (C) Gwl IPs from the same cell lysates of the experiment described in Figure 2E, in which prometaphase-arrested control (Cont.) and Fcp1 siRNAs-treated (Fcp1), as well as Fcp1 siRNAs-treated complemented with wild type Fcp1 (Fcp1WT), HeLa cells were treated – or + RO3306 for 15 min, split into three portions and incubated in kinase reactions at 37°C and with recombinant Ensa protein. Kinase reactions were stopped at indicated time points of incubation and probed for indicated antigens (Mk IPs were incubated for 15 min). Graphs show quantitation (arbitrary units) of pS67-Ensa optical density. Data shown are representative of three independent experiments per type. (D) Schematic for the proposed model of PP2A-B55 control.
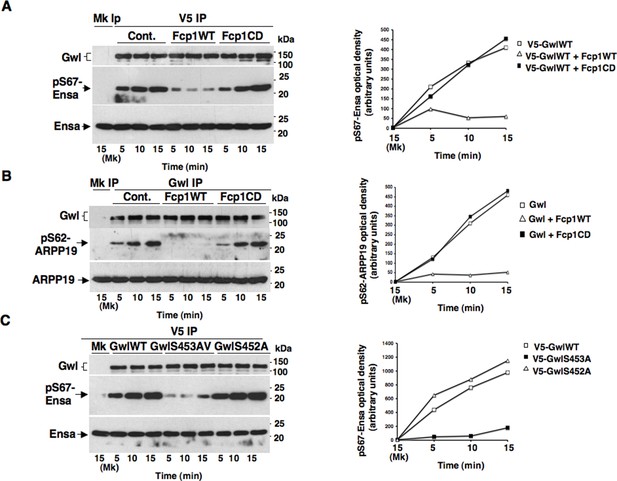
Fcp1 affects Ensa/ARPP19 kinase ability of Gwl.
(A) V5 IP from V5-GwlWT-transfected, prometaphase-arrested, HeLa cells was divided into three sets and incubated for phosphatase reactions with buffer (Cont.), Fcp1WT or Fcp1CD proteins (Mk IP; 1/3 mock IP incubated with buffer). IPs were washed, each set split into three portions and incubated in kinase reactions at 37°C with recombinant Ensa protein. (B) Gwl IP from prometaphase-arrested HeLa cell lysates was divided into three sets. Each set was incubated in phosphatase reactions containinig either just buffer (Cont.), Fcp1WT or Fcp1CD proteins (Mk IP; 1/3 mock IP incubated with buffer). After phosphatase reaction, IPs were washed, each set split into three portions and incubated in kinase reactions at 37°C with recombinant ARPP19 protein. (C) V5 IP from lysates of prometaphase-arrested HeLa cells, previously transfected with V5-GwlWT, V5-GwlS453A or V5-GwlS452A, were split into three portions and incubated in kinase reactions at 37°C with recombinant Ensa protein. Kinase reactions were stopped at indicated time points and probed for indicated antigens (Mk IPs were incubated for 15 min). Graphs show quantitation (arbitrary units) of pS67-Ensa and of pS62-ARPP19 optical density. Data shown are representative of three independent experiments per type.
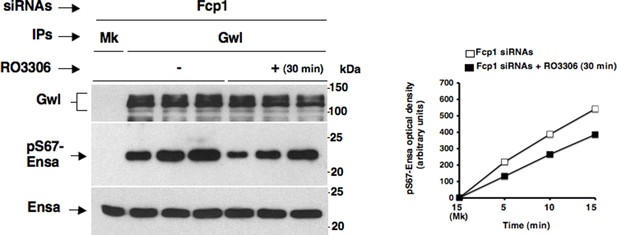
Effects of prolonged Cdk1 inhibition on Gwl activity in Fcp1-depleted cells.
Gwl IPs from cell lysates of prometaphase-arrested Fcp1 siRNAs-treated (Fcp1) HeLa cells treated – or + RO3306 for 30 min, the same cell lysates of the experiment described in Figure 2—figure supplement 2, were each split into three portions and incubated in kinase reactions at 37°C with recombinant Ensa protein. Kinase reactions were stopped at indicated time points of incubation and probed for indicated antigens (Mk IPs were incubated for 15 min). Graphs show quantitation (arbitrary units) of pS67-Ensa optical density. Data shown are representative of three independent experiments per type.





