The MAP kinase pathway coordinates crossover designation with disassembly of synaptonemal complex proteins during meiosis
Figures
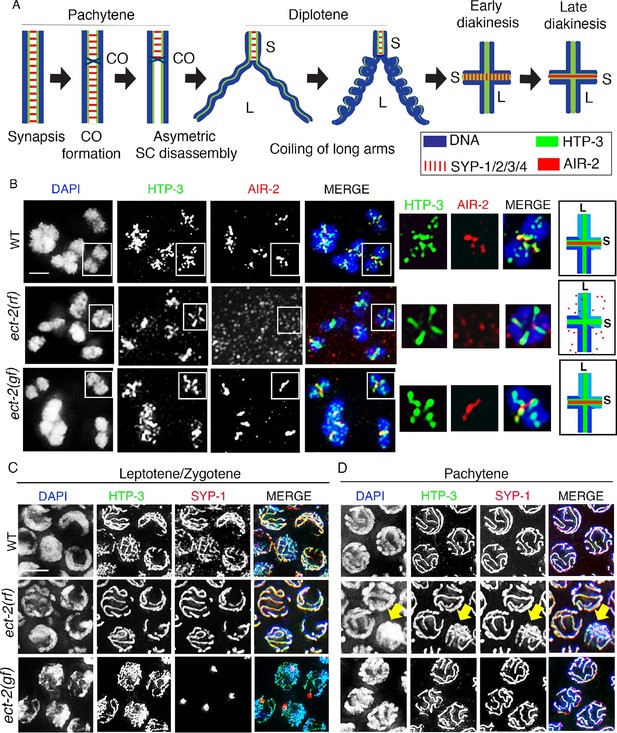
ECT-2 regulates AIR-2 localization and SC dynamics in meiotic prophase I.
(A) Schematic representation of SC dynamics and chromosome remodeling during prophase I of meiosis. A single pair of homologous chromosomes (bivalent) is shown for simplicity. Upon entrance into pachytene, the SC is present along the full length of the pairs of homologous chromosomes. CO formation is completed within the context of fully synapsed chromosomes, and in worms, a single CO is formed per homolog pair usually at an off-centered position. Chromosome remodeling has been proposed to take place around the off-centered CO (or CO precursor) resulting in a cruciform configuration comprised of two intersecting perpendicular chromosomal axes of different lengths (long and short arms of the bivalent, corresponding to the longest and shortest distances from the off-centered CO/CO precursor site to opposite ends of the chromosomes). This remodeling involves disassembly of central region components of the SC (SYP-1/2/3/4) along the long arms of the bivalents starting during late pachytene and diplotene resulting in the restricted localization of these proteins to the short arms. During diplotene and diakinesis, chromosomes undergo condensation as evidenced by a coiling of the arms and increased bivalent compaction. In late diakinesis, the SC proteins located on the short arms are replaced by AIR-2, which promotes the separation of the homologs at the end of meiosis I. CO – crossover, S – short arm and L – long arm. (B) Immunolocalization of HTP-3 and AIR-2 on -1 oocytes at diakinesis in wild type, ect-2(gf) and ect-2(rf) gonads. AIR-2 is localized to the short arm of the bivalents in wild type and ect-2(gf) mutants, but fails to localize onto chromosomes in ect-2(rf) mutants. Diagrams on the right illustrate the cruciform structure of the bivalents at this stage consisting of long (L) and short (S) arms and the localization of AIR-2 (red) and HTP-3 (green) in wild type and ect-2(rf) mutant. White box indicates the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. (C) Immunolocalization of HTP-3 and SYP-1 on leptotene/zygotene nuclei from gonads of the indicated genotypes. SYP-1 aggregates (polycomplexes) are detected in ect-2(gf) mutants. (D) SYP-1 and HTP-3 localize throughout the full length of the synapsed homologous chromosomes during pachytene in wild type and in most pachytene nuclei in ect-2(rf) mutants. Arrowhead indicates a nucleus where chromosomes persist in the DAPI-bright and tighter clustered configuration characteristic of the leptotene/zygotene stage in ect-2(rf). In ect-2(gf) mutants, meiotic nuclei that progressed through the leptotene/zygotene stage before the shift to the non-permissive temperature show wild type-like SYP-1 localization. Worms from all the indicated genotypes, including wild type, were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. n>26 gonads were examined for each genotype in (B) and n>15 in (C) and (D). Bars, 2 μm.
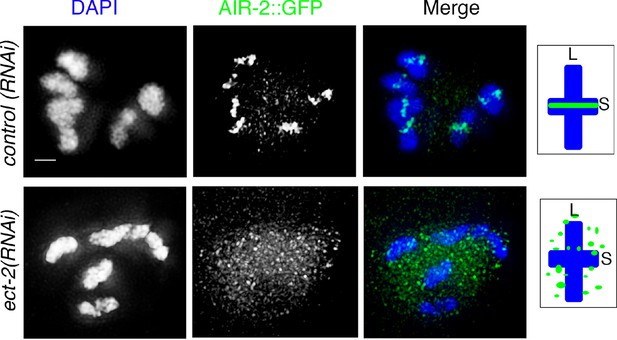
ect-2(RNAi) affects AIR-2::GFP loading on the chromosomes.
AIR-2::GFP localization in -1 oocytes at diakinesis of control(RNAi) and ect-2(RNAi) gonads. AIR-2::GFP localization was visualized using anti-GFP antibody. n>35 gonad arms were analyzed for each. 100% showed a defect in AIR-2::GFP loading on the short arms of the bivalent in oocytes at diakinesis in ect-2(RNAi). Diagrams on the right illustrate the cruciform structure of the bivalents at this stage consisting of long (L) and short (S) arms and the localization of AIR-2 (green) in control(RNAi) and ect-2(RNAi) gonads. Bar, 3 μm.
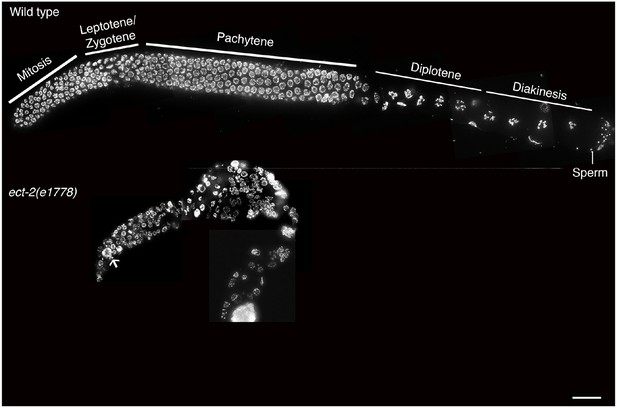
ect-2(e1778) null mutant exhibits disorganized germline with fewer germ cells.
Low-magnification DAPI stained whole gonad arm images of age-matched (A) wild type and (B) ect-2(e1778). ect-2(21778) null mutants exhibit an extremely disorganized germ line with fewer germ cells. White arrow points to abnormal large DAPI bodies in the premeiotic tip indicative of defects in mitotic division. Mitotic defects could mask the role of ect-2 in meiotic progression making it impossible to use the ect-2 null mutant to understand the role of ect-2 in meiosis. n>10 gonads were examined for each genotype. Bar, 10 μm.
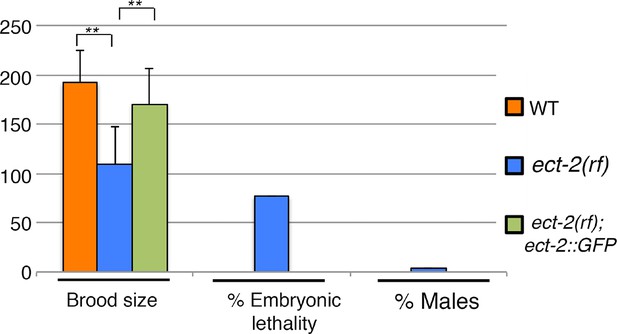
Rescue of ect-2(ax751rf) phenotypes by expression of functional ECT-2::GFP in the germline.
Histogram showing brood size, embryonic lethality and Him (High Incidence of Males) phenotype in wild type, ect-2(ax751rf), and ect-2(ax751rf) mutant animals expressing ECT-2::GFP. ECT-2::GFP is able to rescue brood size, embryonic lethality and the Him phenotype of ect-2(ax751rf) mutants at the non-permissive temperature. **p<0.0001 (unpaired student’s t-test). 20, 30 and 20 gonad arms were analyzed for wild type, ect-2(ax751) and ect-2(ax751); ECT-2::GFP animals, respectively. ECT-2::GFP has been shown to rescue the sterility phenotype of ect-2(gk44) null mutants in Chan and Nance (2013). Brood size indicates the total number of eggs (non-hatched and hatched) laid per hermaphrodite. All worms from the indicated genotypes, except ect-2(ax751);ECT-2::GFP, were grown at 15°C and shifted to 25°C as L4 worms. ect-2(ax751) animals expressing ECT-2::GFP were grown at 20°C to prevent silencing of the transgene and shifted as L4 animals to 25°C. All genotypes were analyzed 18–24 hr post-L4 at 25°C.
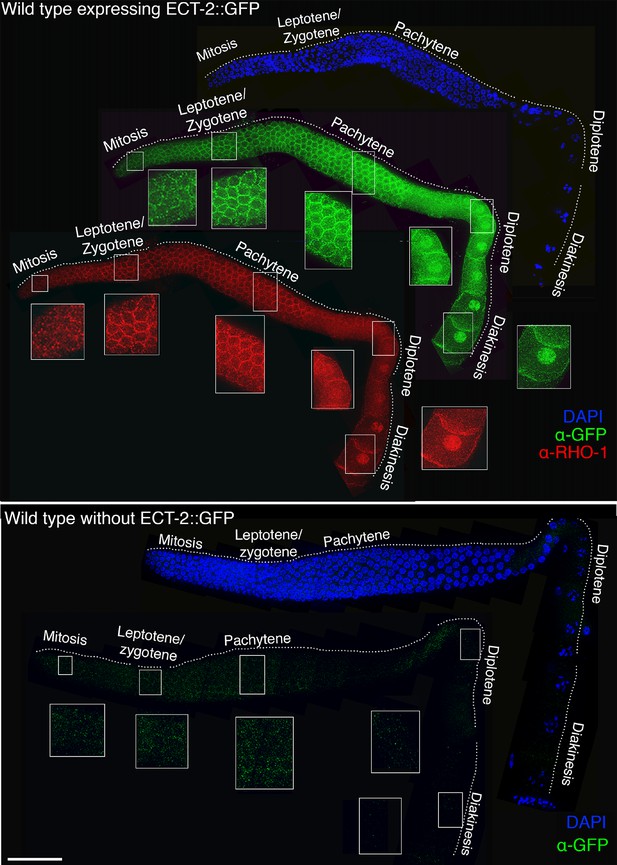
Co-localization of ECT-2::GFP and RHO-1 in the germline.
Low-magnification image of a whole mounted gonad from a wild type animal expressing ECT-2::GFP stained with antibody against GFP (green), RHO-1 (red) and DAPI (blue). ECT-2::GFP and RHO-1 are expressed throughout the germline on the germ cell membrane giving a honeycomb pattern. In addition, ECT-2::GFP and RHO-1 show nuclear localization in late pachytene, diplotene and diakinesis stage nuclei. ECT-2::GFP and RHO-1 co-localize throughout the germ line. A whole mounted gonad from a wild type animal that does not express ECT-2::GFP was stained with anti-GFP antibody as a negative control. Insets show higher-magnification images of indicated regions. 15 gonad arms were analyzed to verify expression pattern. Worms from all the indicated genotypes were grown and analyzed at 20°C. Bar, 20 μm.
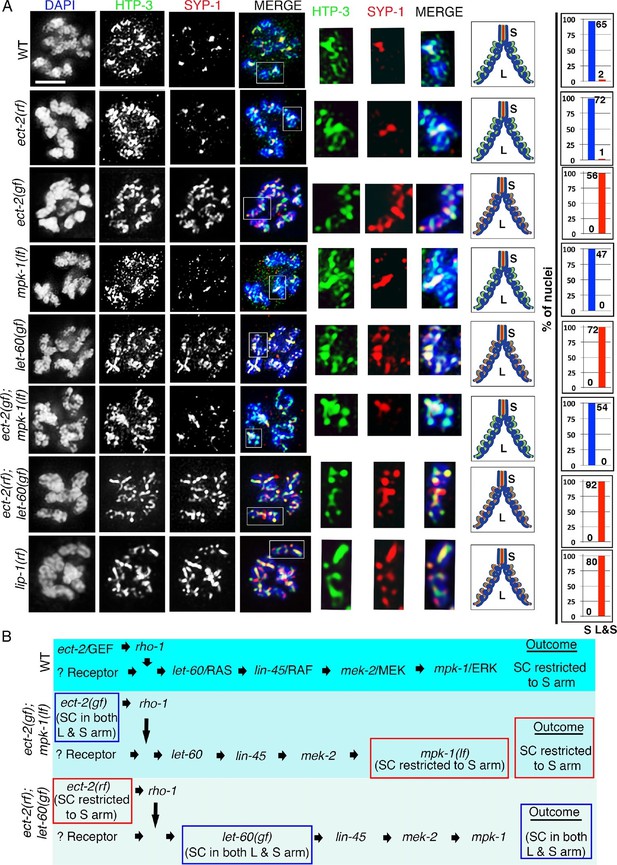
ECT-2 regulates the disassembly of SC proteins from the long arms of the bivalents through the MPK-1 pathway.
Co-staining with HTP-3 (green), SYP-1 (red) and DAPI (blue) of diplotene and early diakinesis nuclei from the indicated genotypes. At diplotene and early diakinesis, SYP-1 localization is restricted to the short arm in both wild type and ect-2(rf) mutants. In contrast, SYP-1 fails to disassemble from the long arm and become restricted to the short arm of the bivalents in ect-2(gf), let-60(gf) and lip-1(rf) mutants. mpk-1(lf) mutants suppress the defect in disassembly of SC proteins from the long arms observed in ect-2(gf) mutants whereas ect-2(rf); let-60(gf) double mutants exhibit the phenotype of let-60(gf) mutants. Illustrations depict the bivalent configuration at this stage. White box indicates the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Worms from all the indicated genotypes, including wild type, were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. Histograms on the right indicate the percentage of diplotene and diakinesis stage nuclei with SYP-1 either only on the short arm (S, blue) or on both long and short arms (red, L&S) of the bivalents. All the bivalents were examined in every nucleus and the bivalents in the same nucleus either all exhibited SYP-1 staining on both the long and short arms or all exhibited staining only on the short arms. Numbers of nuclei scored are shown. (B) Schematic representation shows the crosstalk between the conserved ECT-2 and RAS/MAPK pathways and restriction of the SC to the short (S) arm of the bivalent at diakinesis in wild type. Remaining schematic shows epistasis analysis in ect-2(gf); mpk-1(ga111lf) and ect-2(rf); let-60(gf) double mutants. S indicates short arm and L indicates long arm. n>15 gonads arms were analyzed for each genotype. Bar, 2 μm.
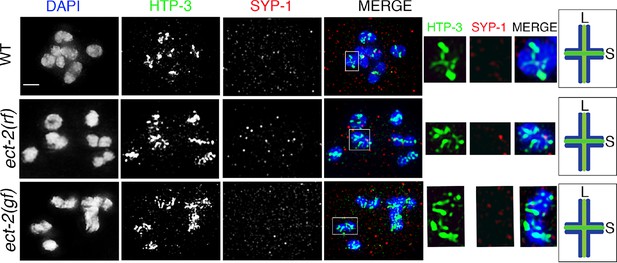
SYP-1 and HTP-3 localization at diakinesis in ect-2 mutants.
Higher-magnification images of -1 oocytes at diakinesis show that HTP-3 and SYP-1 immunolocalization is indistinguishable between wild type, ect-2(ax751rf) and ect-2(zh8gf) mutants. HTP-3 is present along both short and long arms of the bivalents whereas the SC is fully disassembled by the end of diakinesis and SYP-1 is no longer observed on either short or long arms. White boxes indicate the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Illustrations depict the bivalent configuration at this stage. S indicates short arm, L indicates long arm, localization of HTP-3 is in green and DNA is shown in blue. Worms from all the indicated genotypes, including wild type, were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. 15, 21 and 26 gonad arms were analyzed for wild type, ect-2(rf) and ect-2(gf) genotypes, respectively. Bar, 2 μm.
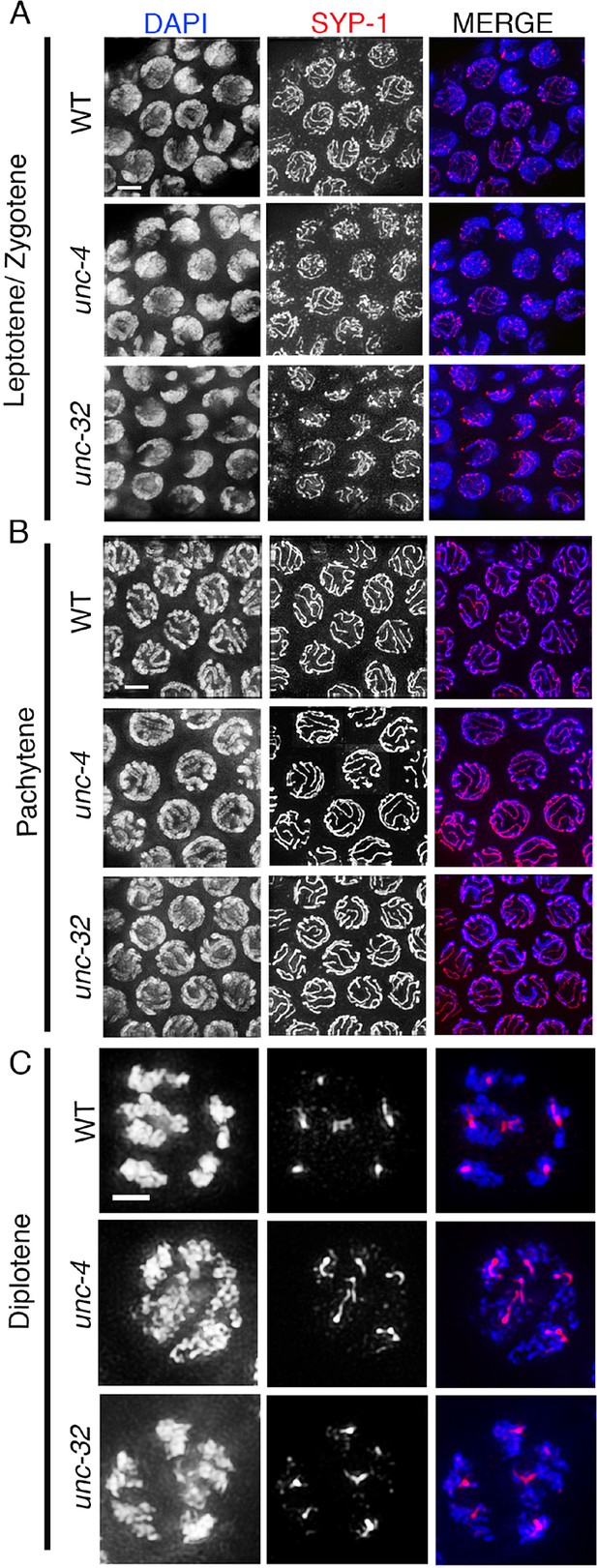
SYP-1 localization in unc-4 and unc-32 mutants.
Higher-magnification images of wild type, unc-4, and unc-32 mutants stained with anti-SYP-1 (red) and DAPI (blue). (A) leptotene/zygotene, (B) pachytene and (C) diplotene stage nuclei were analyzed for SYP-1 localization in wild type, unc-4 and unc-32 mutants. SYP-1 localizes between the homologs at the leptotene/zygotene stage, is observed as continuous tracks between completely synapsed chromosomes at pachytene, and is lost from the long arms and retained at the short arms of the bivalents, as shown in diplotene, in a manner indistinguishable between wild type and unc-4 and unc-32 mutants. 10, 12 and 11 gonad arms were analyzed for wild type, unc-4, and unc-32 mutants, respectively. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. Bar, 2 μm.
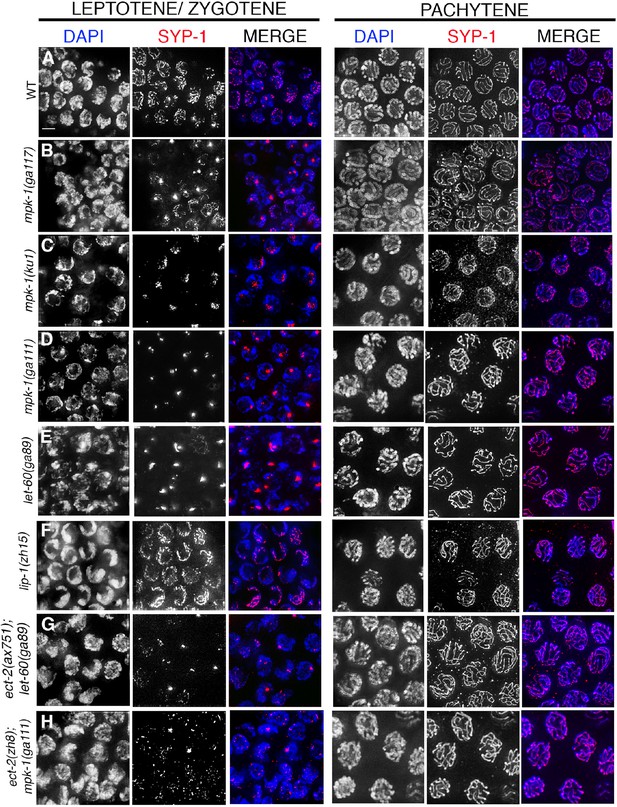
SYP-1 localization in MAP kinase mutants.
Immunolocalization of SYP-1 in leptotene/zygotene and pachytene stage nuclei for the indicated genotypes. Instead of the continuous tracks of SYP-1 observed between homologs in wild type (A), SYP-1 aggregates (polycomplexes) are observed at leptotene/zygotene in (B) mpk-1(ga117), (C) mpk-1(ku1rf), (D) mpk-1(ga111lf), (E) let-60(ga89gf) mutants, (G) ect-2(ax751rf); let-60(ga89gf), and (H) ect-2(zh8gf); mpk-1(ga111lf) mutants. SYP-1 localization is indistinguishable from wild type at this stage in (F) lip-1(zh15rf) mutants. In all these mutants, nuclei that have passed the leptotene/zygotene stage before the shift to the non-permissive temperature exhibit a wild type-like SYP-1 localization pattern at pachytene. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4 for phenotype. n>15 gonad arms were analyzed for each genotype. Bar, 2 μm.
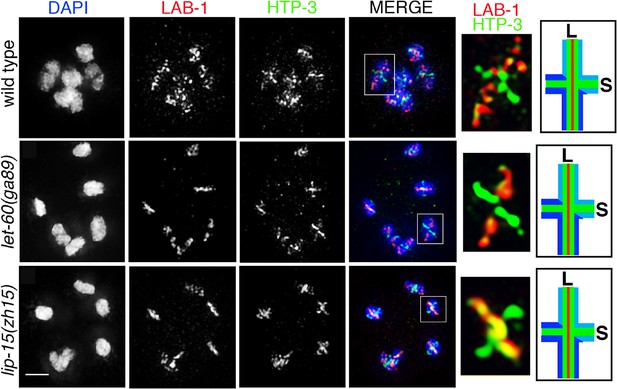
LAB-1 localization in let-60(ga89gf) and lip-1(zh15rf) mutants.
Immunolocalization of LAB-1 and HTP-3 in diakinesis oocytes of the indicated genotypes. LAB-1 is lost from the short arms and restricted to the long arms of the bivalents in a manner indistinguishable from wild type in both let-60(ga89gf) and lip-1(zh15rf) mutants. n>14 gonad arms were analyzed for each genotype. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4 for phenotype. White boxes indicate the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Illustration on the right depicts bivalent at this stage. LAB-1 is indicated in red, HTP-3 in green, DNA in blue, S indicates short arm and L indicates long arm. Bar, 2 μm.
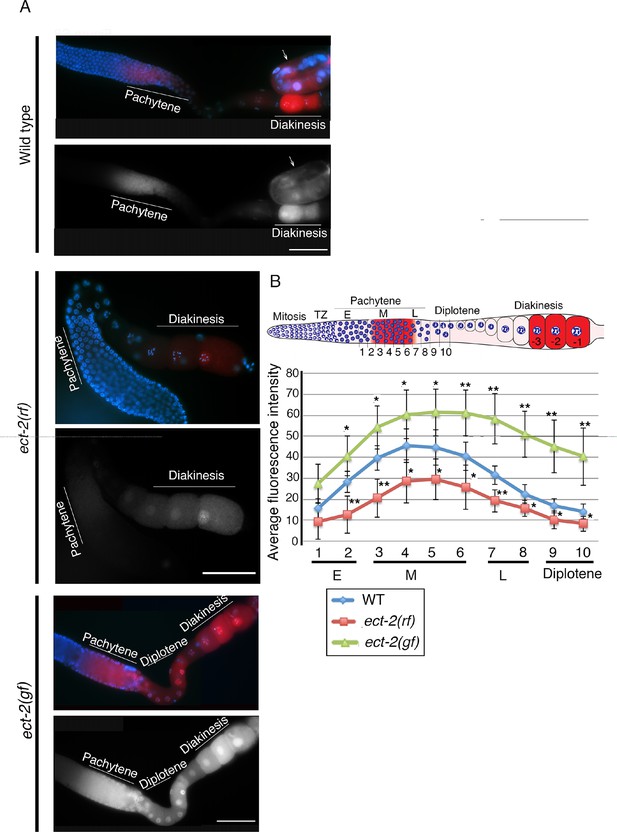
Immunolocalization of dpMPK-1 in the germlines of ect-2 mutants.
(A) Low-magnification images of whole gonad arms showing the immunolocalization of activated MPK-1 (dpMPK-1). ect-2(ax751rf) mutants exhibit reduced dpMPK-1 signal in the pachytene zone compared to wild type. Meanwhile, ect-2(zh8gf) mutants exhibit increased dpMPK-1 signal compared to wild type and it persists from late pachytene through early diakinesis where it is turned off in wild type. Arrow indicates intestine. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4 for phenotype. (B) Graph showing the quantification of dpMPK-1 fluorescence signal intensity in different regions of the gonad arm in wild type, ect-2(ax751rf) and ect-2(zh8gf) mutants. **p<0.005, *p<0.05 (unpaired student’s t-test). Regions where dpMPK-1 signal was quantified are indicated on the diagram depicting the hermaphrodite germline. Progression from mitosis into meiosis is displayed from left to right and the last three oocytes at diakinesis (-3 to -1) are indicated. TZ stand for transition zone (leptotene/zygotene). E, M and L stand for early, mid and late pachytene, respectively. Number on the X-axis of the graph corresponds to the number depicted in the gonad arm where fluorescence intensity was measured. >14 gonads arms were analyzed for each genotype.
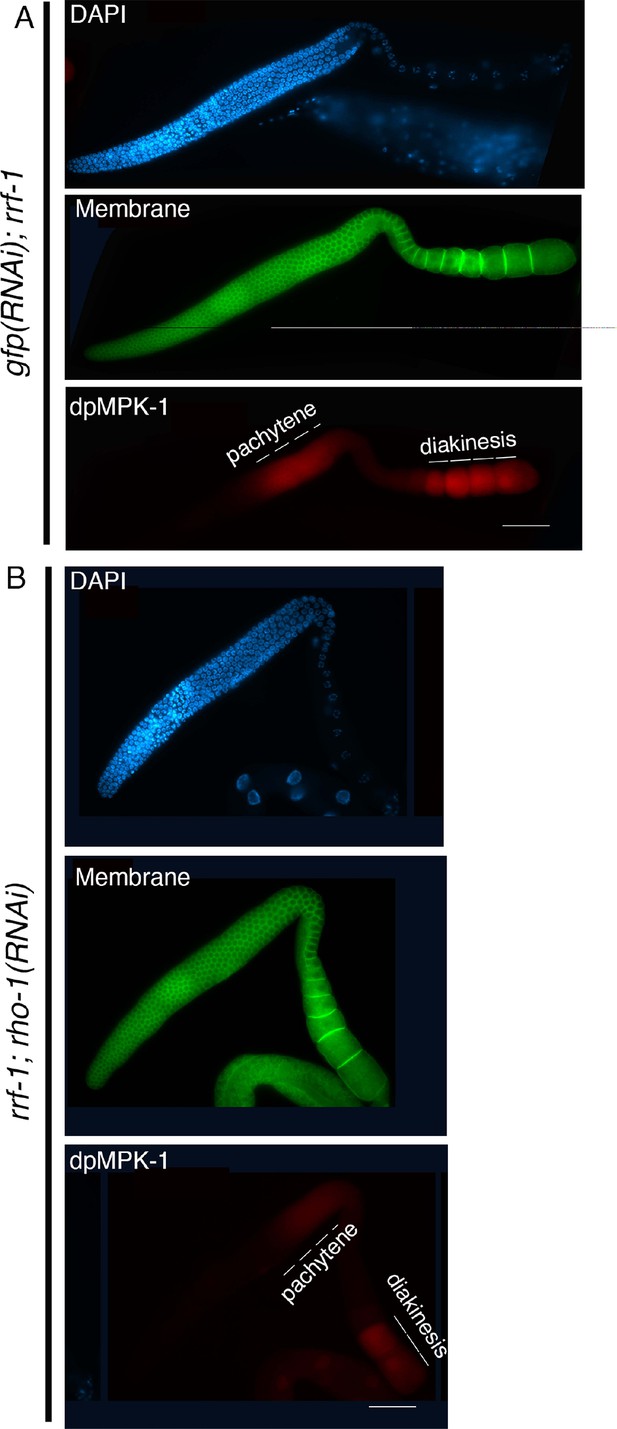
Active dpMPK-1 level is reduced upon rho-1 RNAi in the germline.
Low-magnification images of whole gonad arms image for (A) gfp(RNAi) (control) and (B) rho-1(RNAi) stained with anti-dpMPK-1 (red), anti-SYN-4 (green), and DAPI (blue). SYN-4 marks the germ cell membrane. RNAi was performed in the rrf-1 mutant to specifically knock down rho-1 in the germline. Depletion of rho-1 in the germline reduces active dpMPK-1 signal in the germline compared to the control RNAi. Bars, 20 μm.
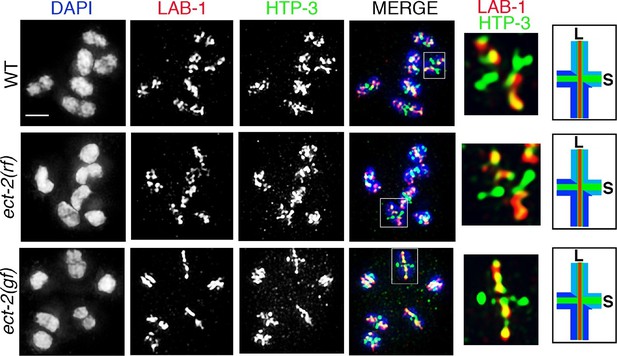
ECT-2 does not alter LAB-1 localization.
Immunolocalization of LAB-1 and HTP-3 in -1 oocytes at diakinesis indicates that LAB-1 is restricted to the long arm of the bivalents in ect-2(rf) and ect-2(gf) mutants similar to wild type. Illustration depicts the cruciform structure of the bivalents at this stage and the localization of LAB-1 (red) to the long arm (L) and HTP-3 (green) to both long and short (S) arms in wild type. White box indicates the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Worms from all the indicated genotypes, including wild type, were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. n>15 gonads were analyzed for each genotype. Bar, 2 μm.
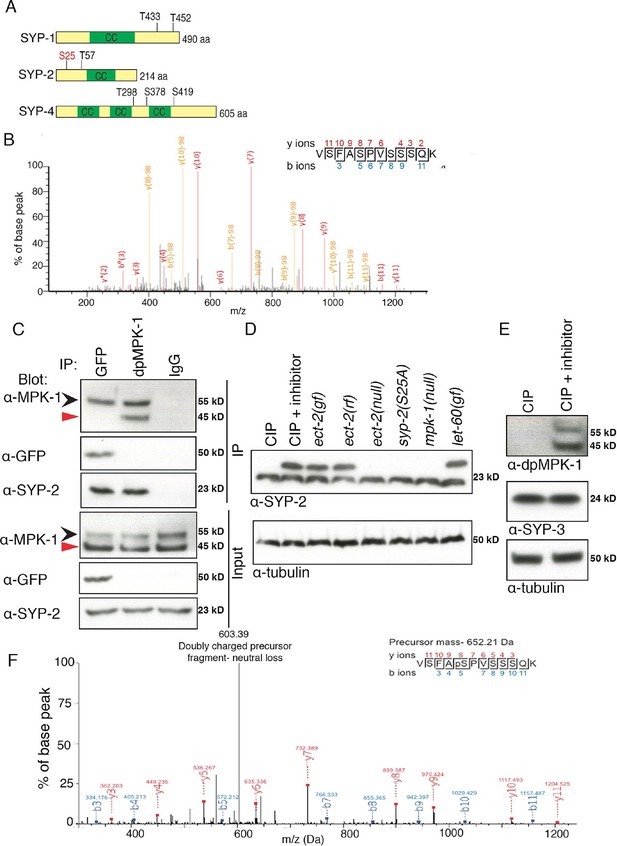
Phosphorylation of SYP-2 is dependent on the MAP kinase pathway.
(A) Schematic representation of SYP-1, SYP-2, and SYP-4 proteins with predicted MAP kinase phosphorylation sites indicated in black and site confirmed by mass spectrometry indicated in red. CC indicates the coiled-coil domains. (B) MS/MS fragmentation spectrum for SYP-2 phosphopeptide VSFASPVSSSQK in the range 100–1300m/z. The annotated spectrum shows fragment ion species matched between theoretical and measured values. 'b ions' are generated through fragmentation of the peptide bond from the N-terminus, whereas 'y ions' are generated through fragmentation from the C-terminus. Ion species detected with a mass loss of 98 (phosphoric acid) are indicated in yellow; those ions without phospho-loss are annotated in red. Analysis of 'y' and 'b' ions with and without phospho-loss is consistent with phosphorylation of the second serine (VSFApSPVSSSQK), corresponding to S25 of the SYP-2 protein. (C) Western blots showing immunoprecipitation of SYP-2 from SYP-2::GFP whole worm lysates with a GFP antibody and dpMPK-1 and IgG immunoprecipitation from wild type whole worm lysates with dpMPK-1 and IgG antibodies, respectively. Standard SDS-PAGE was used and western blots were probed with the indicated antibodies. Black arrow (upper band) indicates germline-specific and red arrow (lower band) indicates soma-specific isoforms of MPK-1. The germline-specific isoform of MPK-1 co-immunoprecipitates with SYP-2 confirming the in vivo interaction detected between dpMPK-1 and SYP-2. (D) Calf intestinal phosphatase assay (CIP) showing SYP-2 is phosphorylated in vivo. After CIP treatment, only a faster migrating band of SYP-2 is present whereas in the presence of the phosphatase inhibitor, both faster and slower migrating bands are present. In the mpk-1(ga117) and ect-2(e1778) null mutant lysates, the upper band is no longer present whereas in let-60(ga89) and ect-2(zh8) gain-of-function mutants, as well as in ect-2(ax751rf) reduction-of-function mutants, both upper and lower bands are present. In the syp-2(S25A) phosphodead mutant, only the lower migrating band is present. α-tubulin is used as a loading control. Worms from all indicated genotypes were grown at 15°C and shifted to 25°C at the L4 stage. Worm lysates were prepared from 18–24 post-L4 worms. Phos-tag SDS-PAGE was used for better separation and detection of phosphorylated proteins. (E) Western blot showing that in the presence of CIP, dpMPK-1 is not present, whereas in the absence of CIP activity dpMPK-1 is present, indicating that the CIP assay is working. There is no mobility shift detected for the SYP-3 protein in either presence or absence of CIP activity. α-tubulin is used as a loading control. Phos-tag SDS-PAGE was used as for (D). (F) In vitro kinase assay showing SYP-2 as a potential direct phosphorylation substrate of ERK MPK kinase. Tandem mass (MS/MS) spectrum of the SYP-2 protein subjected to the in vitro kinase reaction. A SYP-2 peptide containing a phosphorylated serine residue (Ser-25), VSFApSPVSSSQK, was identified in this spectrum. The neutral loss from the doubly charged precursor ion is indicative of the phosphorylated peptide. The b and y product ions are indicated in blue and red, respectively. The spectrum is representative of two independent experiments.
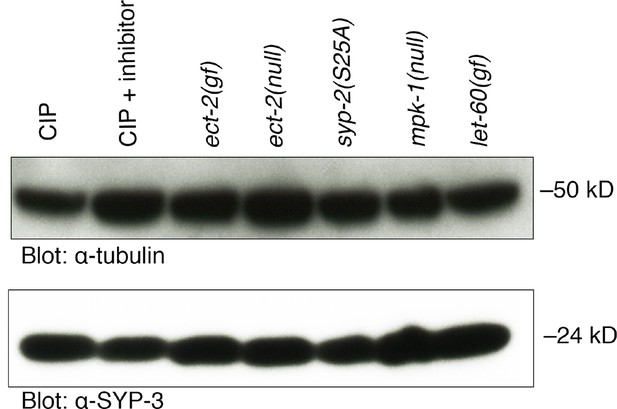
SYP-3 does not exhibit a detectable mobility shift in vivo.
Calf intestinal phosphatase assay (CIP) showing that SYP-3 may not be phosphorylated in vivo. There is no difference in the migration of the SYP-3 band with and without CIP treatment as well as in the indicated mutant backgrounds. Overexposed blots are shown to indicate that there is no other detectable additional band. Phos-tag SDS-PAGE was used for better separation and detection of phosphorylated proteins. α-tubulin was used as a loading control. Worms from all indicated genotypes were grown at 15°C and shifted to 25°C at the L4 stage. Worm lysates were prepared from 18–24 post-L4 worms.
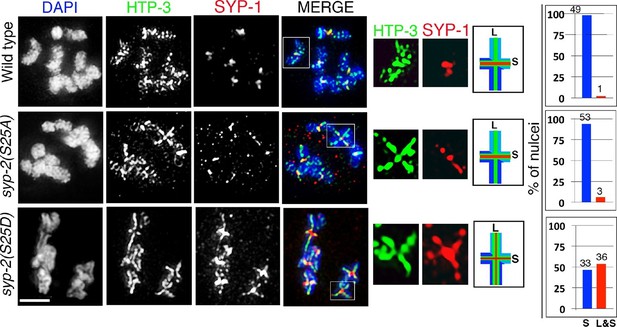
Phosphorylation of SYP-2 at S25 is required for normal SC dynamics.
High-magnification images of wild type, syp-2(S25A) phosphodead, and syp-2(S25D) phosphomimetic mutants co-stained with HTP-3 (green), SYP-1 (red) and DAPI (blue). In the syp-2(S25D) phosphomimetic mutant the SC fails to disassemble from the long arms of the bivalents in 54% of the nuclei scored. All images are of diakinesis nuclei. White box indicates the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Worms from all the indicated genotypes were grown at 20°C and analyzed 18–24 hr post-L4. Histograms on the right indicate the percentage of diplotene and diakinesis stage nuclei with SYP-1 either only on the short arm (S, blue) or on both long and short arms (red, L&S) of the bivalents. All the bivalents were examined in every nucleus and the bivalents in the same nucleus either all exhibited SYP-1 staining on both the long and short arms or all exhibited staining only on the short arms. Numbers of nuclei scored are shown. n>15 gonads were analyzed for each genotype. Bar, 2 μm.
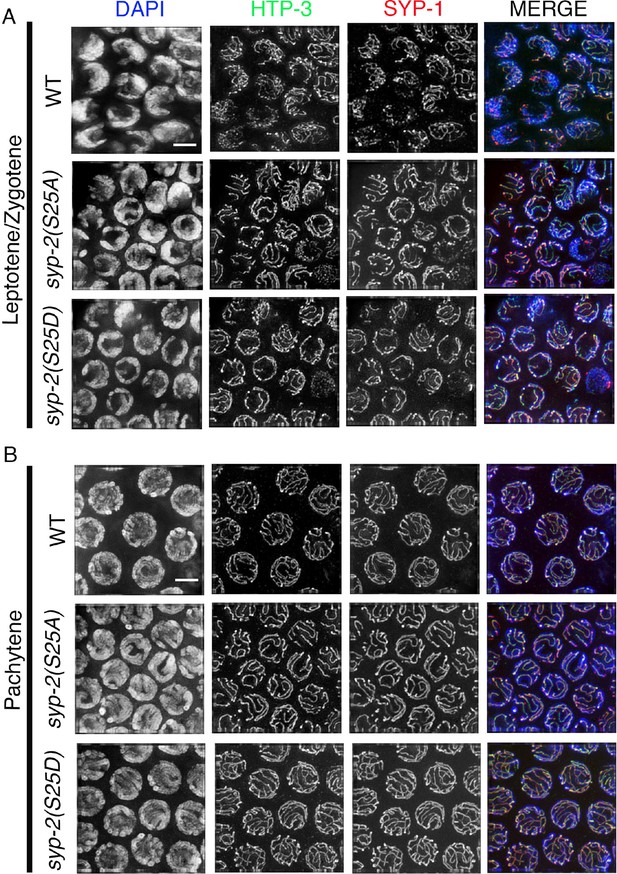
SYP-1 and HTP-3 localization in syp-2 phosphodead and phosphomimetic mutants.
Immunolocalization of HTP-3 and SYP-1 in leptotene/zygotene (A) and mid-pachytene (B) stage nuclei of the indicated genotypes. HTP-3 and SYP-1 localization is indistinguishable between syp-2 phosphodead and phosphomimetic mutants and wild type at these stages. n>15 gonad arms were analyzed for each. Bar, 2 μm.
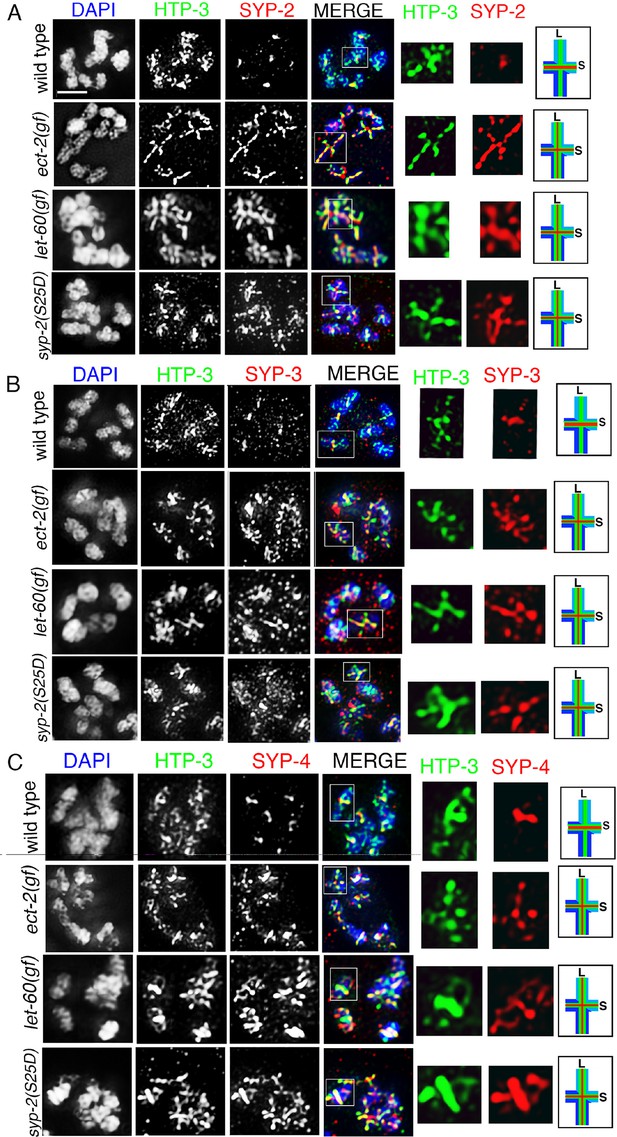
SYP-2, SYP-3 and SYP-4 localization in ect-2(gf), let-60(gf), and syp-2 phosphomimetic mutants.
Higher-magnification images of diakinesis oocytes from the indicated genotypes stained with (A) HTP-3 (green), SYP-2 (red), and DAPI (blue), (B) HTP-3 (green), SYP-3 (red), and DAPI (blue), and (C) HTP-3 (green), SYP-4 (red), and DAPI (blue). White boxes indicate the bivalents shown at a higher magnification on the right. Illustrations depict bivalent at this stage. S indicates short arm and L indicates long arm. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. Bar, 2 μm.
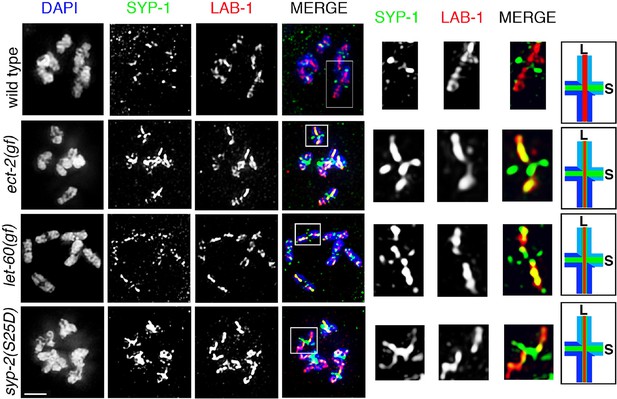
SYP-1 and LAB-1 localization in ect-2(gf), let-60(gf), and syp-2 phosphomimetic mutants.
Higher-magnification images of diakinesis oocytes from the indicated genotypes stained with SYP-1 (green), LAB-1(red), and DAPI (blue). White boxes indicate the bivalents shown at a higher magnification on the right. Illustrations depict bivalents at this stage. SYP-1 is indicated in green, LAB-1 in red, S indicates short arm and L indicates long arm. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. Bar, 2 μm.
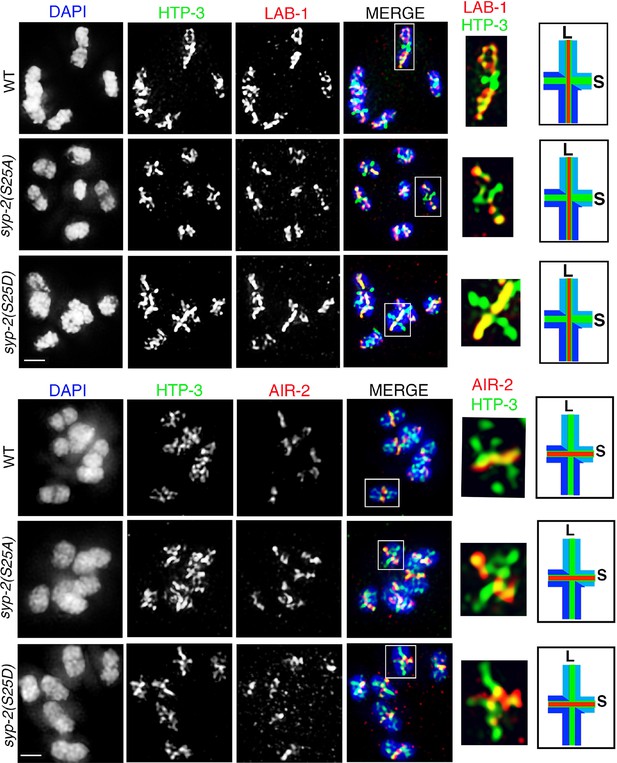
AIR-2 and LAB-1 localization in syp-2 phosphodead and phosphomimetic mutants.
Higher-magnification images of oocytes at diakinesis from the indicated genotypes stained with (A) HTP-3 (green), LAB-1 (red), and DAPI (blue), and (B) HTP-3 (green), AIR-2 (red), and DAPI (blue). White boxes indicate the bivalents shown at a higher magnification on the right. Illustrations depict bivalents at this stage. Worms from all the indicated genotypes were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4. Bar, 2 μm.
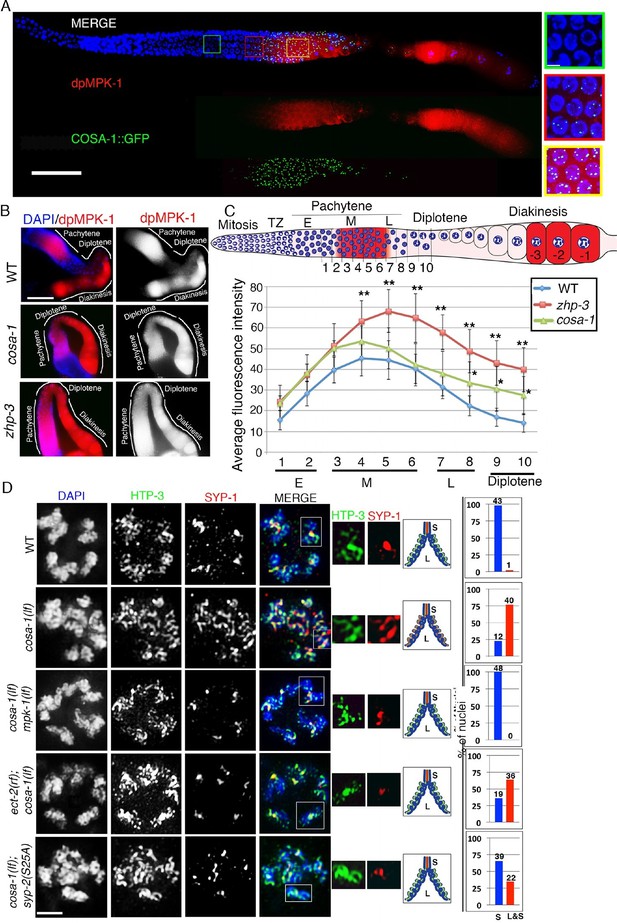
MPK-1 links CO designation with the disassembly of SC proteins.
(A) Low-magnification images of whole mounted gonads depicting COSA-1::GFP and dpMPK-1 localization in wild type. Signals for the pro-crossover marker COSA-1::GFP and dpMPK-1 are both observed at the mid to-late pachytene region. Insets show higher magnification images of different regions in the germline: (green inset) Early to mid-pachytene, no COSA-1 localization and dpMPK-1 is off; (red inset) mid to late-pachytene, COSA-1::GFP starts to localize on the chromosomes concomitant with the appearance of the dpMPK-1 signal; (yellow inset) late pachytene, 6 COSA-1::GFP foci/nucleus are observed and strong dpMPK-1 signal is detected. 20 gonads were analyzed. Bars, 20μm for whole gonad and 2μm for insets. (B) Low-magnification images of wild type, cosa-1 and zhp-3 gonads co-stained with dpMPK-1 (red), and DAPI (blue). dpMPK-1 expression level is not turned off in the cosa-1 and zhp-3 mutants in late pachytene and diplotene in contrast to wild type. n>18 gonads were analyzed for each. Bar, 20 μm. (C) Graph showing the quantification of dpMPK-1 fluorescence signal intensity in different regions of the gonad arm in wild type, zhp-3 and cosa-1 mutants. **p<0.005, *p<0.02 (unpaired student’s t-test; n>18, 17 and 15 gonad arm were analyzed for wild type, zhp-3 and cosa-1 mutants, respectively). Regions where dpMPK-1 signal was quantified are indicated on the diagram depicting the hermaphrodite germline. TZ stands for transition zone (leptotene/zygotene). E, M and L stand for early, mid and late pachytene, respectively. Progression from mitosis into meiosis is displayed from left to right and the last three oocytes at diakinesis (-3 to -1) are indicated. (D) Co-staining with HTP-3 (green), SYP-1 (red) and DAPI (blue) of diplotene nuclei from the indicated genotypes. Illustrations depict the bivalent configuration at this stage. S indicates short arm and L indicates long arm. White boxes indicate the bivalent shown at a higher magnification on the right. Bivalents with both long and short arms clearly displayed were chosen for higher magnification. Histograms on the right indicate the percentage of diplotene and diakinesis stage nuclei with SYP-1 either only on the short arm (S, blue) or on both long and short arms (red, L&S) of the bivalents. All the bivalents were examined in every nucleus and the bivalents in the same nucleus either all exhibited SYP-1 staining on both the long and short arms or all exhibited staining only on the short arms. Numbers of nuclei scored are shown. Bar, 2 μm. Worms from all the genotypes indicated in (A–C) were grown at 20°C and analyzed 18–24 hr post-L4. Worms from all the genotypes indicated in (D) were grown at 15°C, shifted to 25°C at the L4 stage, and analyzed 18–24 hr post-L4.
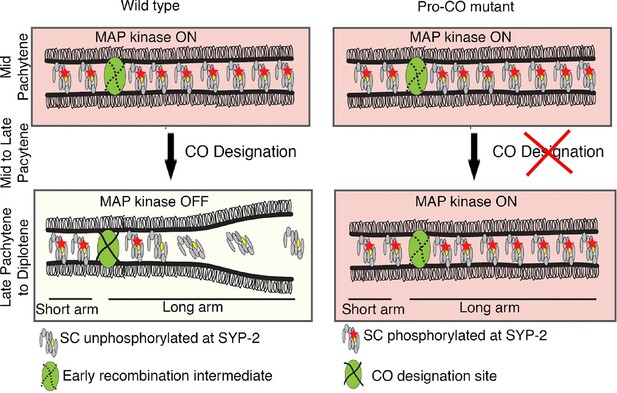
Model for MPK-1-mediated coordination between CO designation and the disassembly of SC proteins during meiosis.
Proposed model for MPK-1 function in coordinating CO designation and the disassembly of SC proteins from the long arms of the bivalents. In mid-pachytene, dpMPK-1 phosphorylates the SYP-2 protein at the S25 site and CO specification is progressing. When CO designation is detected, MAP kinase is turned off, SYP-2 is either dephosphorylated along the long arms of the bivalents by a yet unknown phosphatase or, given the dynamic nature of the SC, phosphorylated SYP-2 is replaced by unphosphorylated SYP-2 in this region and the SC starts to disassemble from the long arms in late pachytene/diplotene. In pro-CO mutants, such as cosa-1 and zhp-3, CO designation is impaired and dpMPK-1 persists at the late pachytene and diplotene regions preventing disassembly of the SC proteins.
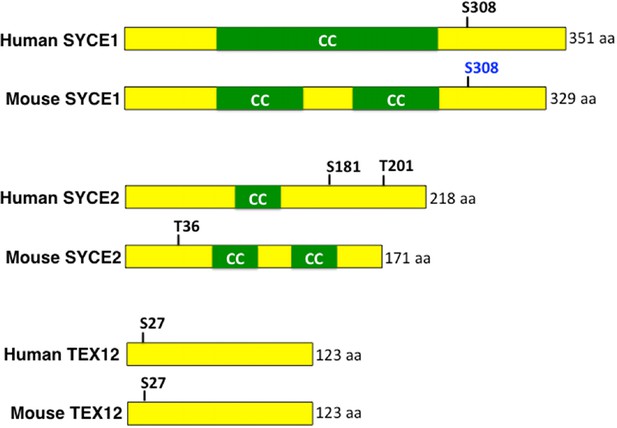
Conserved MAP kinase phosphorylation sites present in central region components of the mammalian SC.
Schematic representation of human and mouse SYCE1, SYCE2 and TEX12 proteins with indicated MAP kinase phosphorylation sites. MAP kinase phosphorylation sites predicted by GSP 2.1 are indicated in black and site verified by mass spectrometry is indicated in blue (Huttlin et al., 2010). CC indicates coiled-coil domains.
Tables
Worms were maintained at 15°C and then shifted to 25°C at the L4 larval stage. All the analyses were conducted at 25°C for all the genotypes indicated above. The 'Eggs Laid' column indicates the average number of eggs laid (including both hatched and non-hatched embryos) per P0 hermaphrodite ± standard deviation. % Embryonic lethality was calculated by dividing the number of non-hatched embryos by the total number of hatched and non-hatched embryos laid. % Males was calculated by dividing the total number of males observed by the total number of hatched (viable) progeny scored. N = total number of P0 worms for which entire broods were scored. N.A.= not applicable.
| GENOTYPE | EGGS LAID | % EMBRYONIC LETHALITY | % MALES | N |
|---|---|---|---|---|
| Wild type | 192 ± 32.3 | 0 | 0 | 20 |
| ect-2(ax751) | 109 ± 38.6 | 76.4 | 3.25 | 30 |
| ect-2(zh8) | 4.5 ± 6.7 | 93.4 | 0 | 32 |
| let-60(ga89) | 3.1 ± 5.6 | 83.8 | 0 | 20 |
| mpk-1(ga111) | 5.8 ± 7.6 | 56 | 0 | 20 |
| ect-2(ax751); let-60(ga89) | 0.7 ± 0.7 | 100 | 0 | 20 |
| ect-2(zh8); mpk-1(ga111) | 0 | N.A. | N.A. | 30 |





