Acquisition of exogenous haem is essential for tick reproduction
Figures
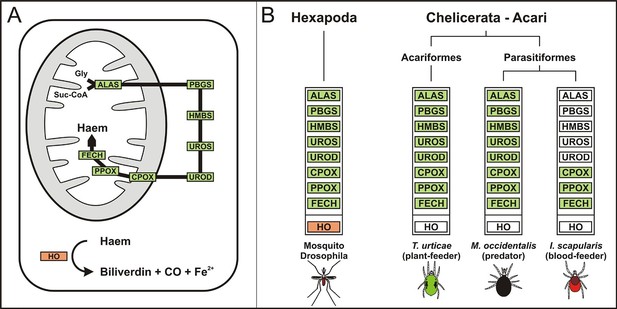
Evolution of haem biosynthetic and degradative pathways.
(A) General scheme of haem biosynthetic and degradative pathways in the eukaryotic cell. Haem biosynthesis (upper) is a series of eight reactions beginning in the mitochondria by condensation of succinyl coenzyme A with glycine, continuing in the cell cytoplasm, and finishing in the mitochondria with the final synthesis of the haem molecule. Haem degradation (lower) is mediated by haem oxygenase in the cell cytoplasm, releasing a ferrous iron, biliverdin, and carbon monoxide. (B) Evolution of haem biosynthetic and degradative pathways in arthropods, according to the available genomic projects. Similarly to vertebrates, hexapods (insects) including blood feeding mosquitoes (red-coloured body), possess all enzymes for haem biosynthesis and degradation. Chelicerates lack haem oxygenase, indicating iron acquisition from sources other than haem. Plant-feeding mites (green-coloured body) of the superorder Acariformes, as well as mite-predating mites (black-coloured body) of the superorder Parasitiformes, possess a complete set of genes for haem biosynthesis. Ticks, which feed solely on blood (red-coloured body) retained only the last three enzymes (mitochondrial) of the pathway. CO - carbon monoxide, Fe2+ - ferrous iron, Gly - glycine, Suc-CoA - succinyl coenzyme A, ALAS - 5-aminolevulinate synthase, PBGS - porphobilinogen synthase, HMBS - hydroxymethylbilane synthase, UROS - uroporphyrinogen synthase, UROD - uroporphyrinogen decarboxylase, CPOX - coproporphyrinogen oxidase, PPOX - protoporphyrinogen oxidase, FECH - ferrochelatase; HO - haem oxygenase. Enzyme nomenclature and abbreviations according to (Hamza and Dailey, 2012)
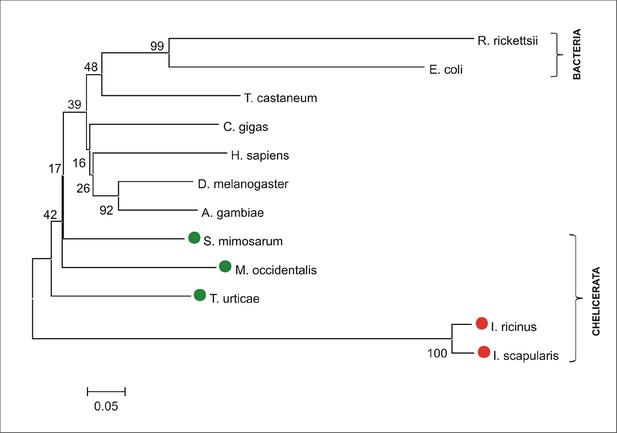
Phylogenetic tree of selected coproporphyrinogen-III oxidases.
Unrooted tree of coproporphyrinogen-III oxidase (CPOX) amino acid sequences reconstructed using the Neighbor Joining method (NJ) based on alignment using ClustalX. The Ixodes scapularis and Ixodes ricinus CPOXs are distant from bacterial, but also from vertebrate, and invertebrate homologues, whose phylogenies cannot be clearly resolved (low bootstrap). Red dots indicate CPOX of ticks and green dots indicate CPOX of other chelicerates. Numbers at branches represent bootstrap supports using NJ criteria with 1000 replicates. The horizontal bar represents a distance of 0.05 substitutions per site. R. ricketsii (Rickettsia rickettsii, bacteria, WP_012151472), E. coli (Escherichia coli, bacteria, WP_001625620), T. castaneum (Tribolium castaneum, red flour beetle, XP_008201513), C. gigas (Crassostrea gigas, pacific oyster, EKC32626), H. sapiens (Homo sapiens, ENSG00000080819), D. melanogaster (Drosophila melanogaster, fruitfly, FBgn0021944), A. gambiae (Anopheles gambiae, malaria mosquito, AGAP004749), S. mimosarum (Stegodyphus mimosarum, social spider, KFM71890), M. occidentalis (Metaseiulus occidentalis, western predatory mite, XP_003744828), T. urticae (Tetranychus urticae, two-spotted spider mite, tetur04g09527), I. ricinus (Ixodes ricinus, castor been tick, JAB79008), I. scapularis (Ixodes scapularis, deer tick, ISCW010977).
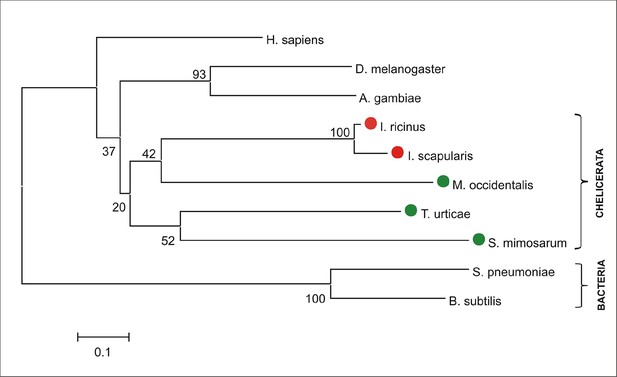
Phylogenetic tree of selected protoporphyrinogen oxidases.
Unrooted tree of protoporphyrinogen oxidase (PPOX) amino acid sequences reconstructed using the Neighbor Joining method (NJ) based on alignment using ClustalX. The Ixodes scapularis, Ixodes ricinus, vertebrate, and invertebrate PPOXs, whose phylogenies cannot be clearly resolved (low bootstraps) are distant from bacterial homologues. Red dots indicate CPOX of ticks and green dots indicate CPOX of other chelicerates. Numbers at branches represent bootstrap supports using NJ criteria with 1000 replicates. The horizontal bar represents a distance of 0.1 substitutions per site. H. sapiens (Homo sapiens, ENSG00000143224), D. melanogaster (Drosophila melanogaseter, fruitfly, FBgn0020018), A. gambiae (Anopheles gambiae, malaria mosquito, AGAP003704), I. ricinus (Ixodes ricinus, castor been tick, JAB84046), I. scapularis (Ixodes scapularis, deer tick, ISCW023396), M. occidentalis (Metaseiulus occidentalis, western predatory mite, XP_003740594), T. urticae (Tetranychus urticae, two-spotted spider mite, tetur10g04900), S. mimosarum (Stegodyphus mimosarum, social spider, KFM82234), S. pneumoniae (Streptococcus pneumoniae, bacteria, CGG00621), B. subtilis (Bacillus subtilis, bacteria, WP_032725328).
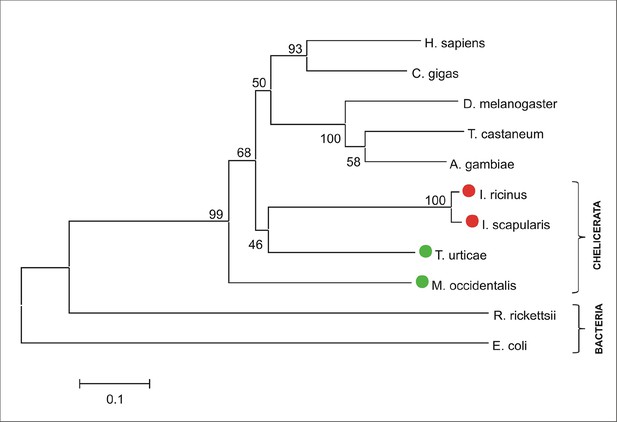
Phylogenetic tree of selected ferrochelatases.
Unrooted tree of ferrochelatase (FECH) amino acid sequences reconstructed using the Neighbor Joining method (NJ) based on alignment using ClustalX. The Ixodes scapularis and Ixodes ricinus FECHs clusters together with other chelicerate homologues. Red dots indicate CPOX of ticks and green dots indicate CPOX of other chelicerates. Numbers at branches represent bootstrap supports using NJ criteria with 1000 replicates. The horizontal bar represents a distance of 0.1 substitutions per site. H. sapiens (Homo sapiens, ENSG00000066926), C. gigas (Crassostrea gigas, pacific oyster, EKC30122), D. melanogaster (Drosophila melanogaster, fruitfly, FBgn0266268), T. castaneum (Tribolium castaneum, red flour beetle, XP_008193416), A. gambiae (Anopheles gambiae, malaria mosquito, AGAP003719), I. ricinus (Ixodes ricinus, castor been tick, JAB74800), I. scapularis (Ixodes scapularis, deer tick, ISCW016187), T. urticae (Tetranychus urticae, two-spotted spider mite, tetur04g02210), M. occidentalis (Metaseiulus occidentalis, western predatory mite, XP_003748486), R. rickettsii (Rickettsia rickettsii, bacteria, WP_012262655), E. coli (Escherichia coli, bacteria, ACI87485).
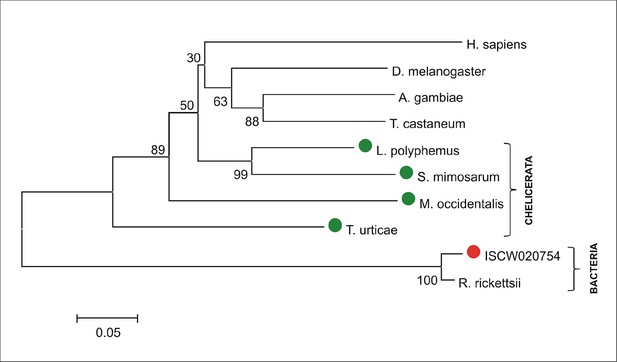
Phylogenetic tree of selected 5-aminolevulinate synthases.
Unrooted tree of 5-aminolevulinate synthase (ALAS) amino acid sequences reconstructed using the Neighbor Joining method (NJ) based on alignment using ClustalX. The ISCW020754 annotated in the Ixodes scapularis genome as a putative serine palmitoyltransferase is clearly a bacterial gene, homologous to Rickettsia, symbionts of ticks. Red dot indicates ISCW020754 sequence from the tick genome, green dots indicate chelicerate ALASs. Numbers at branches represent bootstrap supports using NJ criteria with 1000 replicates. The horizontal bar represents a distance of 0.05 substitutions per site. H. sapiens (Homo sapiens, CAA42916), D. melanogaster (Drosophila melanogaster, fruitfly, CAA74915), A. gambiae (Anopheles gambiae, malaria mosquito, AGAP003184), T. castaneum (Tribolium castaneum, red flour beetle, TC013340), L. polyphemus (Limulus polyphemus, atlantic horseshoe crab, AAD20805), S. mimosarum (Stegodyphus mimosarum, social spider, KFM81891), M. occidentalis (Metaseiulus occidentalis, western predatory mite, XP_003744200), T. urticae (Tetranychus urticae, two-spotted spider mite, tetur32g00320), ISCW020754 (annotated Ixodes scapularis gene, deer tick, ISCW020754), R. ricketsii (Rickettsia rickettsii, bacteria, WP_014363330).
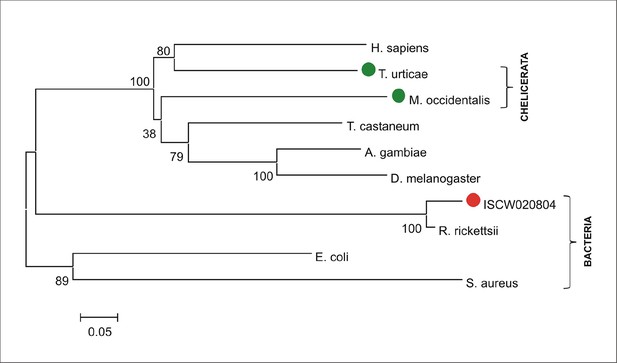
Phylogenetic tree of selected uroporphyrinogen decarboxylases.
Unrooted tree of uroporphyrinogen decarboxylase (UROD) amino acid sequences reconstructed using the Neighbor Joining method (NJ) based on alignment using ClustalX. The ISCW020804 annotated in the Ixodes scapularis genome is clearly a bacterial gene homologous to Rickettsia, symbionts of ticks. Red dot indicates ISCW020804 sequence from the tick genome, green dots indicate chelicerate URODs. Numbers at branches represent bootstrap supports using NJ criteria with 1000 replicates. The horizontal bar represents a distance of 0.05 substitutions per site. H. sapiens (Homo sapiens, NP_000365), T. urticae (Tetranychus urticae, two-spotted spider mite, tetur19g03090), M. occidentalis (Metaseiulus occidentalis, western predatory mite, XP_003740745), T. castaneum (Tribolium castaneum, red flour beetle, XP_972457), A. gambiae (Anopheles gambiae, malaria mosquito, XP_320631), D. melanogaster (Drosophila melanogaster, fruitfly, ACH92415), I. scapularis (Ixodes scapularis, deer tick, ISCW020804), R. ricketsii (Rickettsia rickettsii, bacteria, WP_014362650), E. coli (Escherichia coli, bacteria, WP_000137647), S. aureus (Staphylococcus aureus, bacteria, KLN00580).
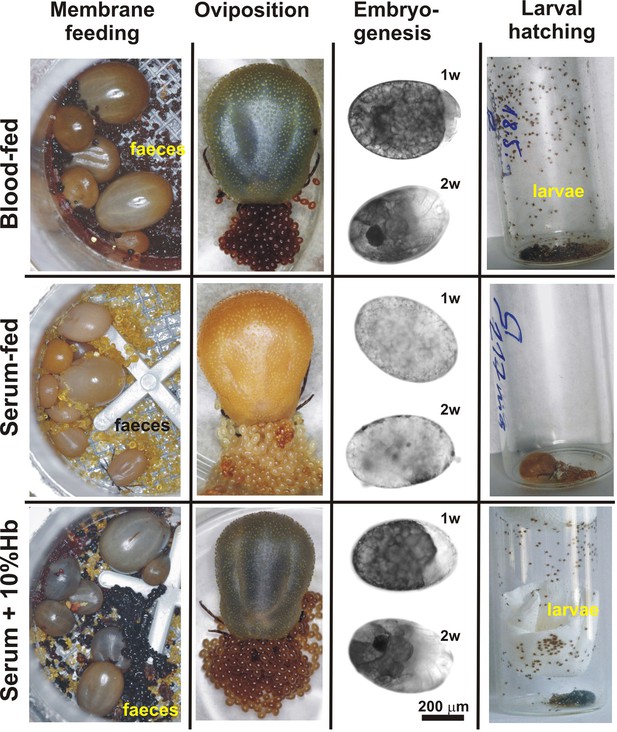
Impact of dietary haemoglobin on tick feeding, oviposition, embryogenesis, and larval hatching.
(Membrane feeding) - membrane feeding in vitro of Ixodes ricinus females on whole blood (Blood-fed), serum (Serum-fed) and on serum supplemented with 10% bovine haemoglobin (Serum + 10% Hb). For dietary composition, see Figure 2—figure supplement 1. (Oviposition) – representative females laying eggs. (Embryogenesis) – microscopic examinations of embryonal development in eggs laid by differentially fed females; 1w, 2w – 1 week, 2 weeks after oviposition, respectively. Note, no embryos developed in eggs from serum-fed ticks, while embryogenesis was rescued in serum + 10% Hb-fed ticks. (Larval hatching) – Laid eggs were incubated to allow larval hatching. Note, no larvae hatched out of eggs laid by serum-fed females and the hatching was fully rescued in serum + 10% Hb-fed ticks. Similar rescue effects were also observed for ticks fed on serum supplemented with 1% and 0.1% Hb (see Figure 2—figure supplement 2)
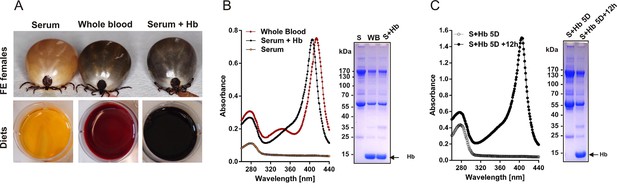
Diets used for tick membrane feeding and faecal examination.
(A) Females of I. ricinus were membrane fed until full engorgement (FE) using whole blood, serum, and serum supplemented with 10% (physiological concentration) of pure bovine haemoglobin (Serum+Hb). (B) Composition of diets. Equal levels of haemoglobin in whole blood and reconstituted Serum+Hb were verified by spectrophotometry (absorbance at ~ 400 nm - Soret peak) and by SDS-PAGE of diets (arrow points to haemoglobin band). (C) Faecal examination. To ensure complete passage of Hb through the digestive tract, faeces were inspected 12 hr after serum supplementation with Hb. Examination of faecal extracts by spectrophotometry (absorbance at ~ 400 nm - Soret peak) and by SDS PAGE confirmed the availability of supplemented Hb before ticks commence a rapid engorgement phase (‘big sip’). Note that the protein profile of faeces was almost identical to that of the applied meal.
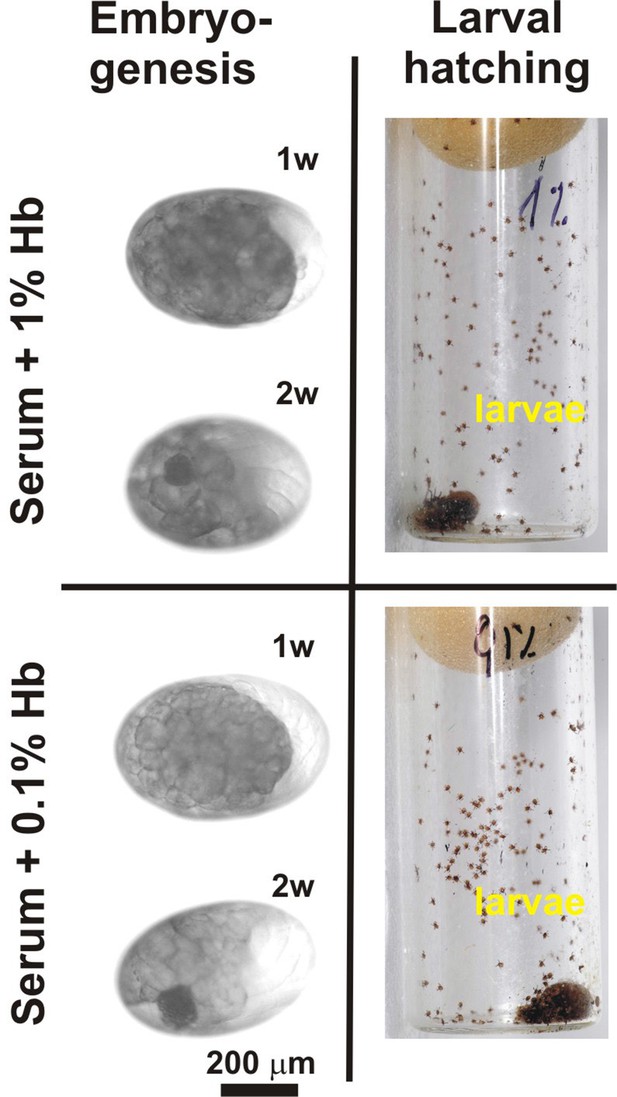
Rescue experiments with sub-physiological levels of haemoglobin.
Embryonal development and larval hatching was fully rescued in I. ricinus females fed on serum supplemented with 1% or 0.1% bovine haemoglobin. (Embryogenesis) – microscopic examination of embryonal development in eggs laid by differentially fed females; 1w, 2w – 1 week, 2 weeks after oviposition, respectively. (Larval hatching) – Laid eggs were incubated to allow larval hatching. Note that tick reproduction was not affected if only one hundredth of the natural haemoglobin concentration was present in the tick diet.
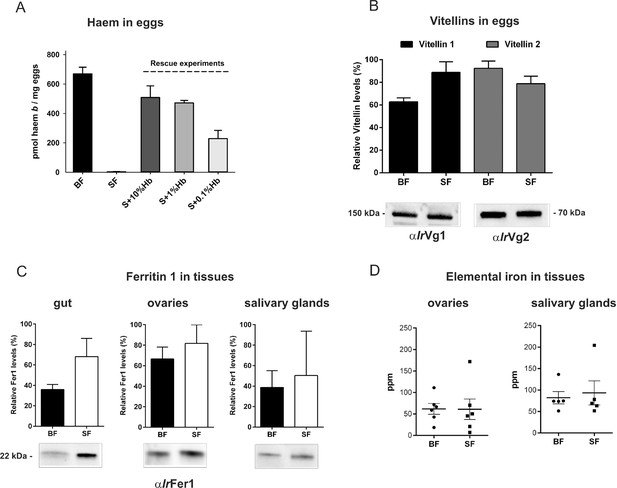
Determination of haemoglobin-derived nutrients in ticks (haem, amino acids, iron).
(A) Levels of haem b were determined by HPLC in egg homogenates from ticks fed on whole blood (BF) serum (SF), and serum supplemented with 10%, 1% or 0.1% bovine haemoglobin (S+10%Hb, S+1%Hb and S+0.1% Hb, respectively; rescue experiments). Data (mean values ± SEM) were acquired from homogenates of three independent clutches of eggs. Representative chromatograms detecting haem b in egg homogenates are shown for BF ticks, SF ticks, and S+10% Hb - fed ticks, see Figure 3—figure supplement 1. (B) Quantitative Western blot analyses detecting levels of vitellin 1 and vitellin 2 in egg homogenates using antibodies raised against vitellin precursors - vitellogenins (IrVg1, IrVg2). Bar charts depict the mean levels ± SEM of the particular vitellin in the egg homogenates from three different clutches of BF ticks or SF ticks (see Figure 3—figure supplement 2). Representative Western blot detection is shown below the bar chart. (C) Quantitative Western blot analyses detecting ferritin1 (IrFer1) in the gut, ovary, and salivary gland homogenates from BF and SF ticks. Bar charts depict the mean ± SEM levels of IrFer1 in the tissue homogenates prepared from three independent tissue pools (see also Figure 3—figure supplement 2). Representative Western blot detections for guts, ovaries and salivary glands are shown below the bar charts. (D) GF-AAS elemental analysis of iron in ovaries and salivary glands pools. Each data point represents a pool of five tissues dissected from BF and SF partially engorged ticks (fed for 6 days). Iron content is expressed in ppm (ng Fe per mg of dry tissue). Main and error bars indicate group means and SEM, respectively.
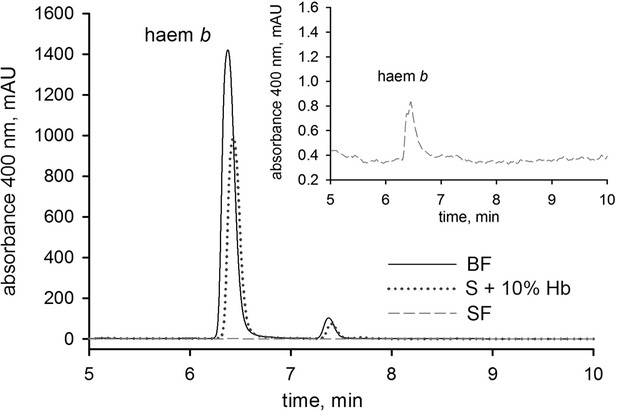
HPLC analysis of haem b in tick egg homogenates.
Homogenates prepared from 10 mg of eggs were collected from three independent egg clutches laid by I. ricinus females fed on different diets. Representative chromatograms are shown detecting haem b in egg homogenates of ticks fed on the whole blood (BF) and haemoglobin-free serum (SF) and serum supplemented with 10% of haemoglobin (S+10% Hb). The inset shows the zoom-in of haem b detection in SF ticks; note the different y-axis scales. For details, see Material and methods.
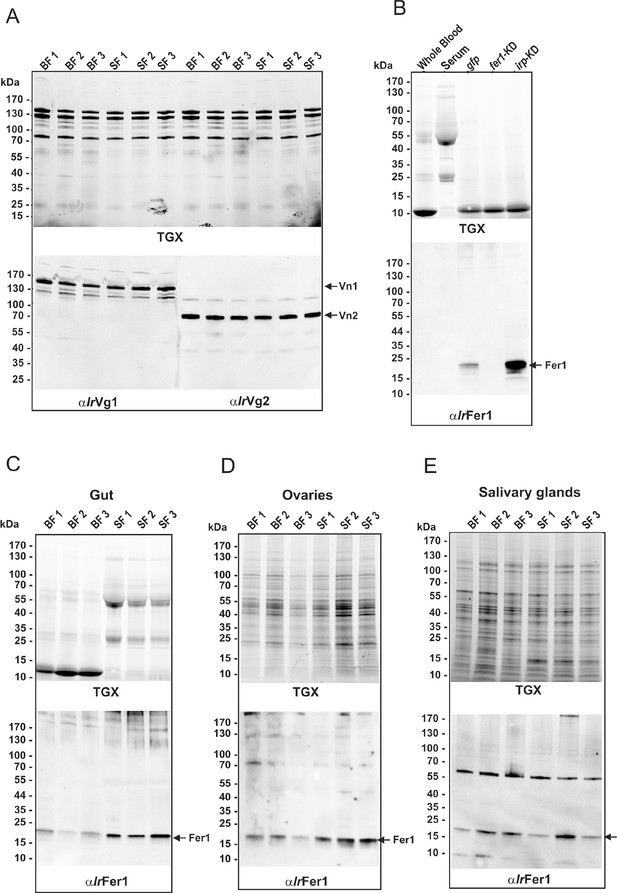
Full appearance of SDS-PAGE and Western blot analyses shown in the Figure 3.
SDS-PAGE analyses were carried out on homogenates prepared from three independent tissue preparations from ticks fed on whole blood (BF) or serum (SF). Protein profiles were visualized using the TGX Stain-Free technology (TGX) and the BioRad ChemiDoc MP imager. (A) SDS-PAGE of homogenates of freshly laid eggs, and Western blot analyses detecting levels of vitellin 1 (Vn1) and vitellin 2 (Vn2) using specific antibodies against vitellogenin 1 (αIrVg1) and vitellogenin 2 (αIrVg2). (B) Control SDS-PAGE (upper panel) and corresponding Western blot (lower panel) for identification of ferritin 1 (Fer1) by RNAi. Western blot analysis of Fer1 levels was performed using specific antibodies against recombinant I. ricinus ferritin1 (αIrFer1). No cross-reacting band was present in the whole blood or serum diet. The Fer1-specific band was clearly present in gut homogenates from naturally fed ticks, pre-injected with gfp dsRNA (gfp) but completely absent in ticks pre-injected with ferritin 1 dsRNA (fer1-KD). RNAi-mediated silencing of iron-regulatory protein (irp-KD) caused a marked Fer1 up-regulation. (C–E) SDS-PAGE (upper panels) and corresponding Western blots (lower panels) used for quantification of Fer1 levels in tissue homogenates dissected from partially engorged BF and SF ticks (fed for 6 days).
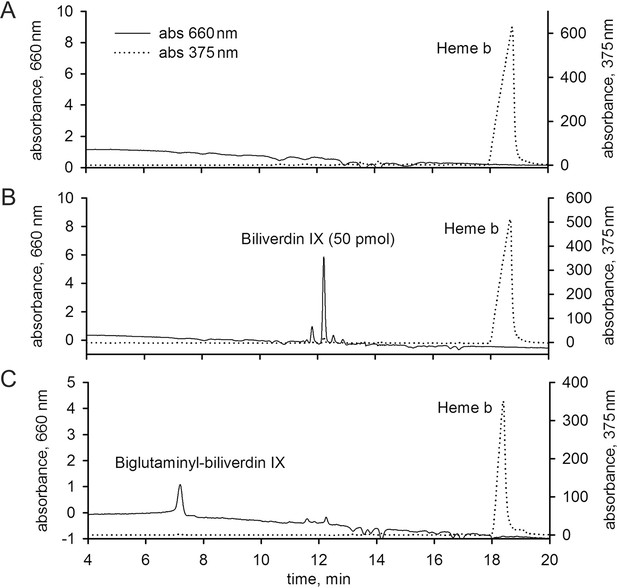
Detection of biliverdin IX derivatives in Ixodes ricinus and Aedes aegypti.
The HPLC using a diode array detector was set to enable a simultaneous determination of haem b and biliverdin IX compounds at wavelengths of 375 nm and 660 nm, respectively. For details, see Materials and methods. (A) I. ricinus gut extracts from fully engorged females 5 days after detachment from the host. (B) I. ricinus gut extracts from fully engorged females 5 days after detachment from the host and spiked with 50 pmol of biliverdin IX standard. (C) Whole body extracts from naturally fed Aedes aegypti mosquitoes allowed to digest blood for three days were used as a positive control. The presence of biglutaminyl-biliverdin IX as a haem b degradation product (Pereira et al., 2007) was detected.
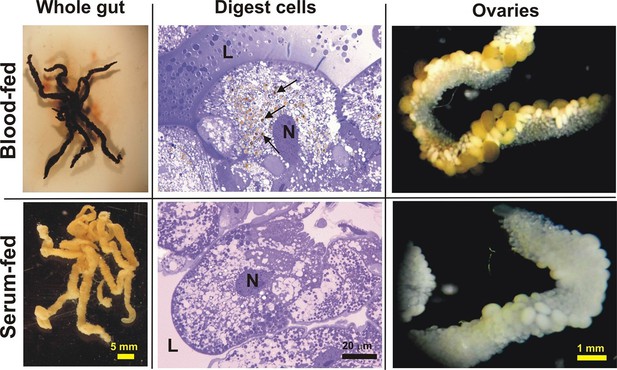
Appearance of the tick gut, digest cells, and ovaries from blood- and serum-fed ticks.
Whole guts from blood-fed (BF) and serum-fed (SF) partially engorged females (fed for 6 days) were dissected and semi-thin sections of digest cells were prepared and stained with toluidine blue. L - lumen; N - nucleus; arrows point to developing haemosomes that were present only in digest cells of BF ticks. Ovaries were dissected from BF and SF fully engorged females 6 days after detachment from the membrane.
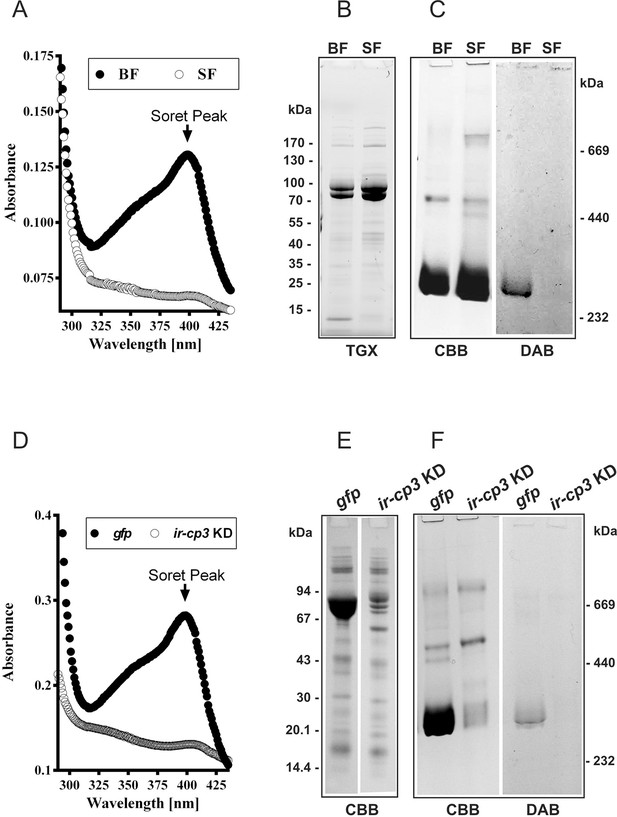
IrCP3 is the major haem-binding protein in I. ricinus haemolymph.
(A-C) Ir-CP3 and haem levels in haemolymph collected from blood-fed (BF) and serum-fed (SF) partially engorged females. (A) Absorbance spectra of haemolymph samples from BF and SF females. (B) SDS-PAGE of haemolymph samples from BF and SF ticks. Protein profiles were visualized using the TGX Stain-Free technology (TGX). (C) Native pore-limit PAGE of heamolymph proteins stained with Coomassie (CBB) and specific co-detection of haem using peroxidase reaction with 3,3´-diaminobenzidine (DAB). (D-F) Effect of RNAi-mediated silencing of ir-cp3 on the Ir-CP3 and haem levels in tick haemolymph. Unfed I. ricinus females were injected with gfp dsRNA (gfp, control group) or with ir-cp3 dsRNA (ir-cp3 KD group) and ticks were allowed to feed naturally on guinea pigs until partial engorgement (fed for 6 days). (D) Absorbance spectra of haemolymph samples from from gfp control and ir-cp3 KD silenced ticks. (E) SDS-PAGE of haemolymph proteins (10 µl, 1:20 dilution) collected from gfp control and ir-cp3 KD ticks. Protein profiles were stained with Coomassie (CBB). (F) Native pore-limit PAGE of heamolymph proteins from gfp control and ir-cp3 KD ticks. Protein profiles were stained with Coomassie (CBB) and haem was co-detected using DAB.
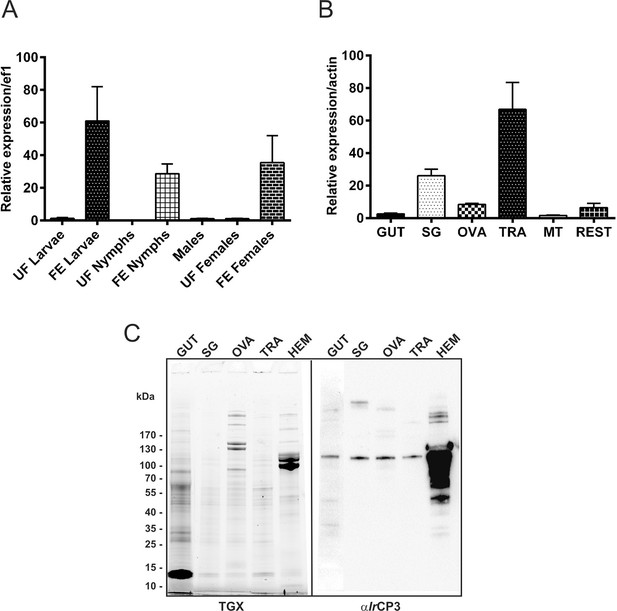
Stage and tissue expression of I. ricinus haemolymph carrier protein (IrCP3).
(A) qPCR analyses of ir-cp3 expression in developmental stages of I. ricinus. (B) qPCR analyses of ir-cp3 expression in tissues dissected from fully engorged females. Data were obtained from three independent cDNA sets, and normalised to elongation factor 1 (ef1) or actin. UF - unfed; FE - fully engorged; SG - salivary glands, OVA - ovaries; TRA - trachea-fat body complex; MT - Malpighian tubules; REST - remaining tissues. (C) SDS-PAGE separation of tissues dissected from I. ricinus females 6 days after detachment, visualized using the TGX Stain-Free technology (TGX), and corresponding Western blot analyses of IrCP3 detected with specific antibodies (αIrCP3). SG - salivary glands, OVA - ovaries; TRA - trachea-fat body complex; HEM - haemolymph. Gut homogenate (50 µg of protein) or other tissue homogenates (10 µg of protein) were loaded per lane.
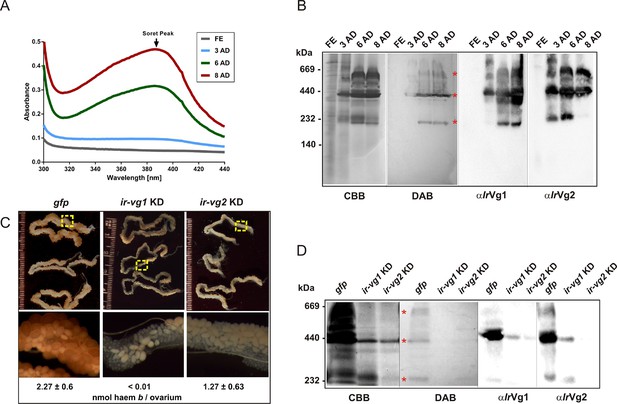
Vitellins are the major haem-binding proteins in tick ovaries.
(A-B) Haem accumulation in tick ovaries occur concurrently with the appearance of vitellins. Ovaries were dissected from I. ricinus females at subsequent time-points after detachment (AD) from the host: FE - fully-engorged; 3 AD, 6 AD, 8 AD - 3, 6, and 8 days AD, respectively. (A) Absorbance spectra of ovaries homogenates show gradually increasing Soret peak following the 3rd day AD. (B) Native pore-limit PAGE of ovaries homogenates stained with Coomassie (CBB), co-detection of haem-associated peroxidase activity with 3,3´-diaminobenzidine (DAB), and Western blot analyses of vitellogenin 1- and vitellogenin 2- cleavage products (αIrVg1 and αIrVg2, respectively). Note that the native IrVg1- and IrVg2-specific bands correspond to the positions of the major haemoproteins in tick ovaries (red asterisks). (C-D) RNAi-mediated silencing of I. ricinus vitellogenin 1 and 2. Unfed I. ricinus females were pre-injected with gfp dsRNA (control, gfp), ir-vg1 dsRNA (ir-vg1 KD), and ir-vg2 dsRNA (ir-vg2 KD), allowed to feed naturally on guinea pigs and then re-injected after detachment from the host with the same amount of dsRNA. (C) Effect of I. ricinus vitellogenin 1 and 2 RNAi-mediated silencing on ovaries appearance and haem levels. Bottom panels show the detailed view of ovary parts depicted by the yellow dashed squares above. Levels of haem b were determined by HPLC in three independent homogenates of ovaries dissected from each tick group 6 days after detachment. (D) Native pore-limit PAGE of ovaries homogenates (10 μg protein per lane) dissected 6 days AD from control (gfp), ir-vg1 KD and ir-vg2 KD ticks. Gels were stained with Coomassie (CBB) for proteins, 3,3´-diaminobenzidine for peroxidase activity of haem (DAB, red asterisks), and Western blot analyses were performed with antibodies against vitellogenin 1 (αIrVg1) and vitellogenin 2 (αIrVg2).
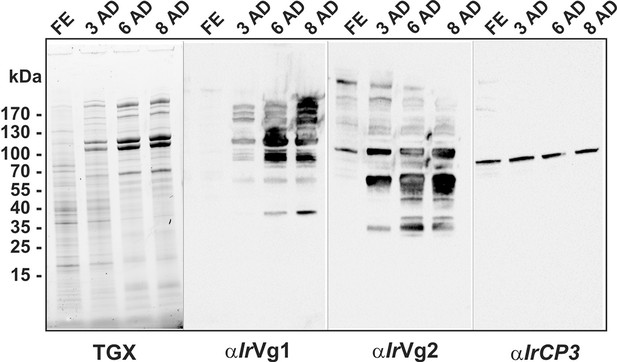
SDS-PAGE and Western blot analyses of ovary homogenates from I. ricinus.
Ovaries were dissected from I. ricinus females at subsequent time-points after detachment (AD) from the host: FE - fully-engorged; 3 AD, 6 AD, 8 AD - 3, 6, and 8 days AD, respectively. Protein profiles of ovaries homogenates were visualised using TGX Stain-Free technology (TGX) and corresponding Western blots of vitellogenin 1-, vitellogenin 2-derived cleavage products and IrCP3 were detected using αIrVg1, αIrVg2, and αIrCP3 specific antibodies, respectively. Note the gradual increase in both vitellins but a constant level of Ir-CP3.
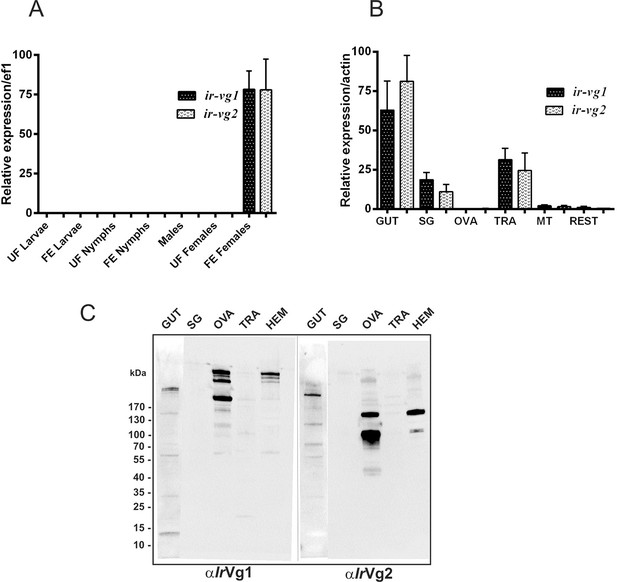
Stage and tissue expression of I. ricinus vitellogenin 1 (IrVg1) and vitellogenin 2 (IrVg2).
Stage and tissue expression of I. ricinus vitellogenin 1 (IrVg1) and vitellogenin 2 (IrVg2). (A) qPCR analyses of ir-vg1 and ir-vg2 expression in developmental stages of I. ricinus. (B) qPCR analyses of ir-vg1 and ir-vg2 expression in tissues dissected from ticks 4 days after detachment. Data were obtained from three independent cDNA sets, and normalized to elongation factor 1 (ef1) or actin. UF - unfed; FE - fully engorged; SG - salivary glands, OVA - ovaries; TRA - trachea-fat body complex; MT - Malpighian tubules; REST - remaining tissues. (C) Western blot analyses of IrVg1, and IrVg2 detected with specific antibodies (αIrVg1), and (αIrVg2), respectively. SG - salivary glands, OVA - ovaries; TRA - trachea-fat body complex; HEM - haemolymph. Gut homogenate (50 µg of protein) or other tissue homogenates (10 µg of protein) were loaded per lane.
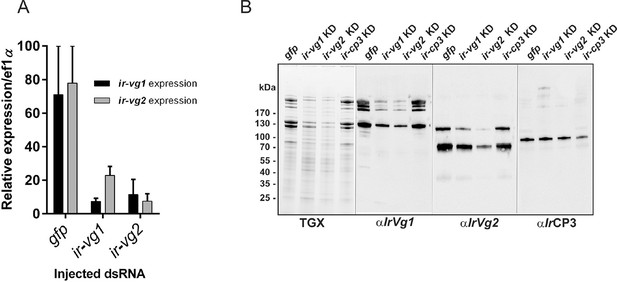
RNAi-mediated silencing of I. ricinus vitellogenin 1 and 2.
Unfed I. ricinus females were pre-injected with gfp dsRNA (gfp), ir-vg1 dsRNA (ir-vg1 KD), ir-vg2 dsRNA (ir-vg2 KD), and ir-cp3 dsRNA (ir-cp3 KD), allowed to feed naturally on guinea pigs and re-injected immediately after detachment (AD) with the same amount of dsRNA. Tissues were dissected 6 days AD. (A) qPCR analysis of ir-vg1 and ir-vg2 gene expression in the tick gut upon RNAi-mediated silencing. Note the mutual co-silencing of both genes. (B) SDS-PAGE protein profiles and corresponding Western blot analyses of ovary homogenates dissected from gfp, ir-vg1 KD, ir-vg2 KD, and ir-cp3 KD ticks. Proteins were visualized using the TGX Stain-Free technology (TGX), and Western blots of IrVg1, IrVg2, and IrCP3 were detected with specific antibodies (αIrVg1), (αIrVg2), and (αIrCP3), respectively. Note the mutual co-silencing of IrVg1 and IrVg2 at the protein level.
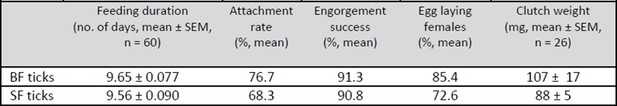
Table 1: Overview of the feeding and egg laying parameters of membrane-fed I. ricinus females.
https://doi.org/10.7554/eLife.12318.026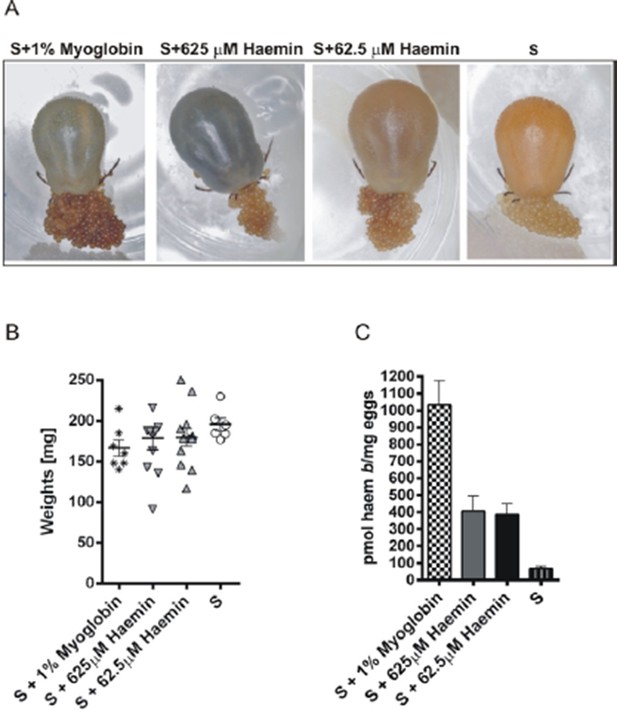
Experimental feeding in vitro of I. ricinus females on serum supplemented with myoglobin or haemin.
Ticks were membrane fed in vitro on bovine serum (S). Pure equine myoglobin (Sigma, M0630) or haemin (Sigma, H9039) of specified concentrations were added to the serum diet from the 6th day of feeding and feeding was then resumed until tick full engorgement. The fully engorged females were weighed, allowed to lay eggs, and haem concentrations in eggs were determined by HPLC. (A) – representative females fed on respective diets laying eggs; note the different female coloration due to distinct amounts of haem in the diet, yet egg clutches are similarly coloured. (B) Weights of fully engorged females fed on respective diets; each symbol presents the weight of one fully engorged female; bar charts depict the mean ± SEM. (C) Levels of haem b were determined by HPLC in egg homogenates from ticks fed on sera supplemented with 1% w/v myoglobin, 625 μM haemin, 62.5 μM haemin, or pure serum. Data (mean values ± SEM) were acquired from homogenates of three independent clutches of eggs. For further details see the Material and Method section.
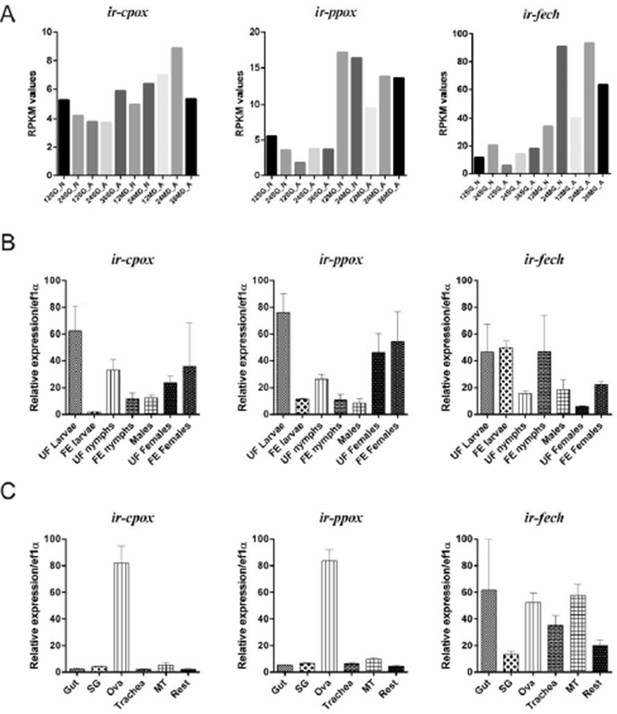
Expression of coproporphyrinogen-III oxidase (ir-cpox), protoporphyrinogen oxidase (ir-ppox), and ferrochelatase (ir-fech) in the tick Ixodes ricinus.
(A) Expression profiles of nymphal and female adult I. ricinus stages over initial phases of feeding from available RNAseq data (Kotsyfakis et al., 2015). SG – salivary glands; MG – gut; N – nymph; A – adult; 12, 24, 36 – 12, 24, 36 hours of feeding. (B) qPCR analyses of ir-cpox, ir-ppox, and ir-fech expression profiles over active developmental stages of I. ricinus and (C) over tissues dissected from partially-engorged females fed for 6 days (C). Data were obtained from three independent cDNA sets, and normalized to elongation factor 1α (ef1α). UF – unfed; FE – fully engorged; SG – salivary glands, OVA – ovaries; Trachea – trachea-fat body complex; MT – Malpighian tubules; Rest – remaining tissues. Mean values +/- SEM are shown.
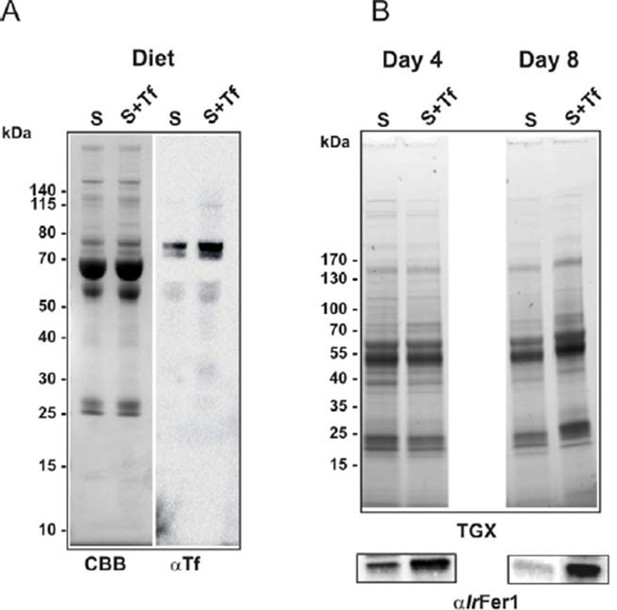
Increased transferrin levels in serum led to higher levels of ferritin 1 in tick gut.
Ticks were fed on serum or on serum supplemented with 3 mg/ml of bovine holo-Transferrin (Sigma, T1283). This addition increased the concentration of transferrin in serum approximately 2-fold, whereas the amount of transferrin iron was increased 3‒4 fold (iron saturation of natural transferrin in serum is usually about 30%). (A) SDS PAGE of diets (10 µg per lane) used for the experiment: S – serum; S+Tf – serum with added 3 mg/ml of iron-saturated transferrin, stained with Coomassie blue (CBB) and Western blot with anti-transferrin specific antibodies (αTf). (B) SDS PAGE of gut homogenates dissected on the 4th day of feeding (Day 4) and from fully engorged females (Day 8) visualized using the TGX Stain-Free technology (TGX) and Western blot detection of tick ferritin 1 levels using specific antibodies against recombinant I. ricinus ferritin1 (αIrFer1).
Additional files
-
Supplementary file 1
Prediction of genes coding for haemoproteins in the genome of I. scapularis and their putative function in tick metabolism.
- https://doi.org/10.7554/eLife.12318.023
-
Supplementary file 2
Design, sequences, and sequence similarities of recombinant Vitellogenin_N domains of IrCP3, IrVg1, and IrVg2 used for raising specific antibodies.
- https://doi.org/10.7554/eLife.12318.024
-
Supplementary file 3
Oligonucleotides used in this work.
- https://doi.org/10.7554/eLife.12318.025





