The Arabidopsis transcription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence
Figures
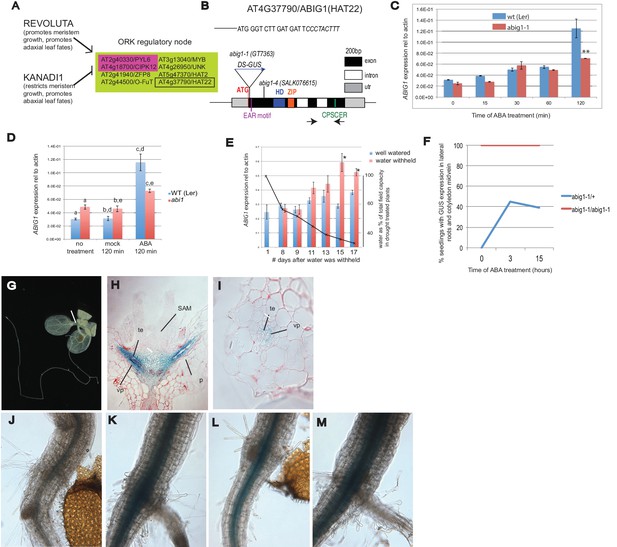
Control of Arabidopsis Abscisic Acid Insensitive Growth 1 (ABIG1/HAT22) expression.
(A) Set of eight ORK genes (OPPOSITELY REGULATED BY REVOLUTA AND KANADI). (B) Structure of the ABIG1 gene. HD - DNA binding homeodomain, ZIP-leucine zipper responsible for homodimerization, EAR – domain responsible for interaction with TOPLESS family co-repressors, CPSCER – conserved domain of unknown function. Arrows - position of PCR primers for ABIG1 Q-RTPCR in panels C-E. Position of gene trap insertion is shown. The sequence above indicates junction between ABIG1 gene and DS insertion (italics). (C) ABIG1 mRNA in wild type and abig1-1 homozygous mutant plants in response to ABA treatment (whole plants grown in liquid media). **p<0.01. (D) ABIG1 mRNA in wild type and abscisic acid insensitive (abi1-1) mutant plants in response to ABA treatment (whole plants grown in liquid media). Letters indicate pairs showing significant differences (p<0.05; t-test). (E) ABIG1 mRNA in response to drought treatment. Water was withheld from rosette stage plants beginning on day one. RNA is from stems and leaves of plants grown in soil. Asterisk indicates time points where watered and unwatered plants differ significantly (p<0.05; t test). (In all cases, bars indicate ± s.e.m. for three biological replicates. The data in each graph are from independent experiments.) (F). Increase in extent of GUS expression in abig-1/+ plants treated with ABA. (G) GUS expression from GT7363 genetrap in abig1-1/ + plant. White line points to area of expression at basal petiole and subtending the shoot apex. (H) Longitudinal thin section through abig1-1/+ seedling showing GUS expression along vascular strands. SAM – shoot apical meristem and associated young leaf primordia; vp – vascular parenchyma; te – tracheary elements; p – petiole. (I) Cross section through a leaf petiole of an abig1-1/+ seedling showing GUS expression in vascular parenchyma cells associated with tracheary elements of xylem. (J) GUS stained hypocotyl/root junction of abig1-1/+ seedling. (K) GUS stained hypocotyl/root junction of abig1-1/+ seedling treated with ABA for three hours. (L) GUS stained hypocotyl/root junction of abig1-1/abig1-1 seedling. (M) GUS stained hypocotyl/root junction of abig1-1/abig1-1 seedling treated with ABA for three hours.
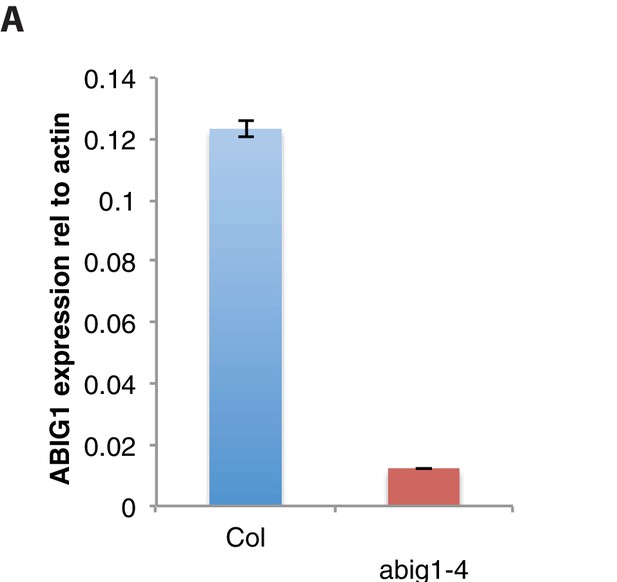
ABIG1 mRNA levels are reduced in homozygous abig1-4 mutants.
(A) ABIG1 mRNA in wild type (Col-0) and abig1-4 mutant plants.
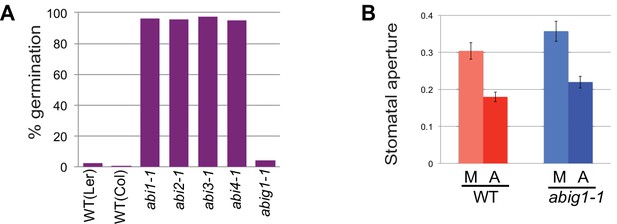
Stomatal closure and germination of abig1-1 mutant seedlings in response to exogenous ABA.
(A) Germination of wild type (wt) and mutant seeds on MS medium with 5 microMolar ABA. Germination of abi1-1, abi2-1, abi3-1 and abi4-1 mutants is ABA resistant while germination of wild type and abig1-1 seedlings is ABA sensitive. (B) Stomatal closure induced by ABA (A) or mock (M) treatment is similar in wild type and abig1-1 mutant plants.
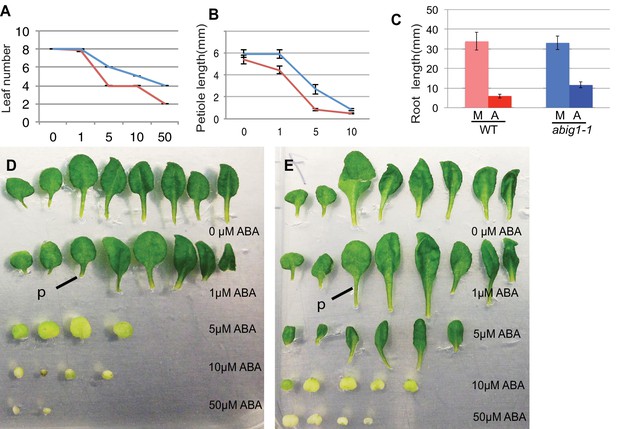
Vegetative growth of abig1-1 mutant seedlings in response to exogenous ABA.
One week old wt and abig1-1 mutant plants were transferred to the ABA containing medium of varying concentration (x axis is in microMolar). Leaf number (A), petiole length of leaf three (B) and primary root length (C) were measured after 14 days on ABA. Red – wild type Ler; blue – abig1-1. Representative wild type (D) and abig1 plants (E) grown on increasing concentration of ABA. Each row of leaves is from a single plant with the first formed leaf to the left. p = petiole of leaf three.
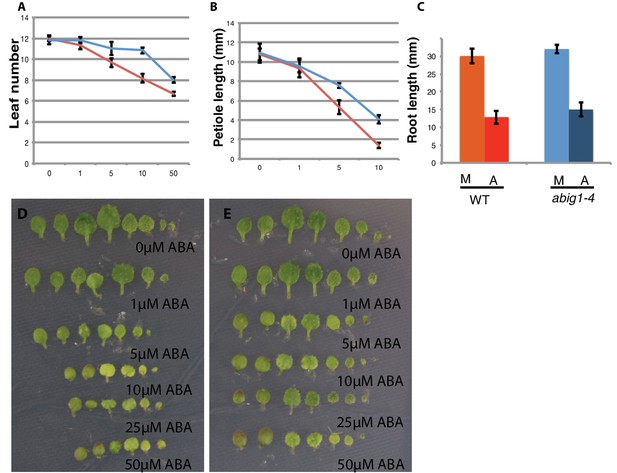
Response of abig1-4 mutants to exogenous ABA.
(A–C) One week old wt (Col-0) and abig1-1 mutant plants were transferred to ABA containing medium of varying concentration. Leaf number (A), petiole length of leaf three (B) and primary root length (C) were measured after 10 days on ABA. Red – wild type, Col-0; blue – abig1-4. (D–E) Representative wild type, Col-0 (D) and abig1-4 plants (E) grown on increasing concentration of ABA. Each row of leaves is from a single plant with the first formed leaf to the left.
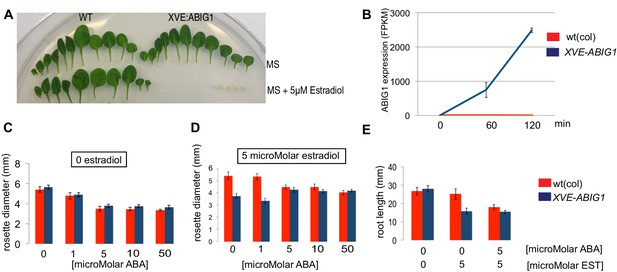
Induced overexpression of ABIG1 mimics ABA treatment.
(A) Wild type plants (left) grown on standard MS plates (upper row) and on plates containing 5 microMolar estradiol (lower row). Each row is a series of leaves taken from a single rosette (first made leaves are to the left). XVE: ABIG1 plants (right) grown on standard MS plates (upper row) and on plates containing 5 microMolar estradiol (lower row). (B). ABIG1 mRNA levels following estrogen induction of XVE:ABIG1. (C) Rosette diameter in wild type (red) and XVE: ABIG1 plants (blue) treated with increasing concentrations of ABA. (D) Rosette diameter in wild type and XVE: ABIG1 plants treated with 5 microMolar estradiol and with increasing concentrations of ABA. (E) Length of the primary root in wild type and XVE:ABIG1 plants treated with 5 microMolar estradiol and with 5 microMolar ABA.
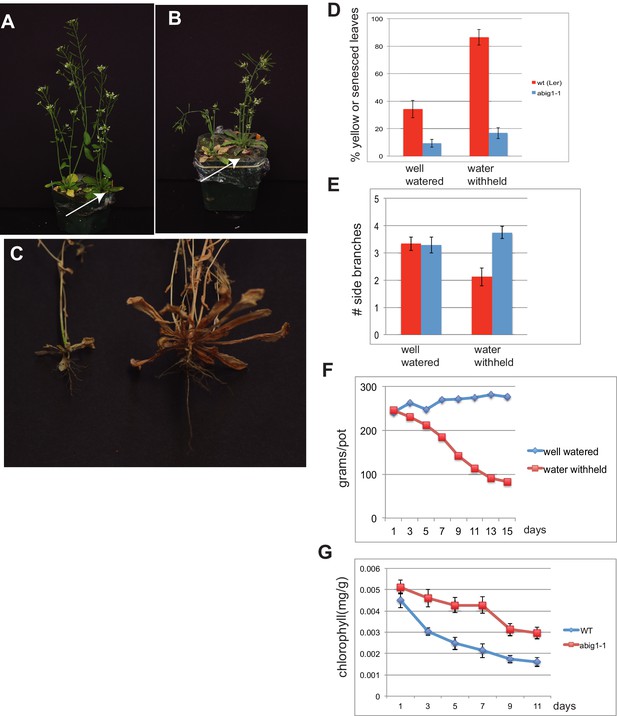
abig1-1 mutants are resistant to drought.
(A) Well watered plants. Wild-type Ler (left) and abig1-1 (right) plants grown in a shared pot. (B) Plants from which water was withheld for 17 days. Wild-type (left) and abig1-1 (right) plants grown in a shared pot. Note yellow, senesced rosette leaves and drooping of wild type plant. (C) Root systems of wild type (left) and abig1-1 (right) plants from which water was withheld. Plants were harvested at the end of their lifecycle at which point both genotypes are fully senesced. (D) Percentage of leaves that had turned yellow after 17 days of withheld water. Total leaves averaged 9 for wild-type and 10 for mutant plants. (E) Number of branches formed in plants from which water was withheld. A branch was scored as present if longer than 1 cm. (F) Rate of water loss from pots during the water withholding period. (G) Chlorophyll content of leaves number 3–8 in drought treated wt and abig1-1 mutant plants over the course of the experiment. This experiment (10 pairs of mutant and Ler wild-type) is a replicate of the experiment shown in A–E (16 pairs of mutant and Ler wild-type).
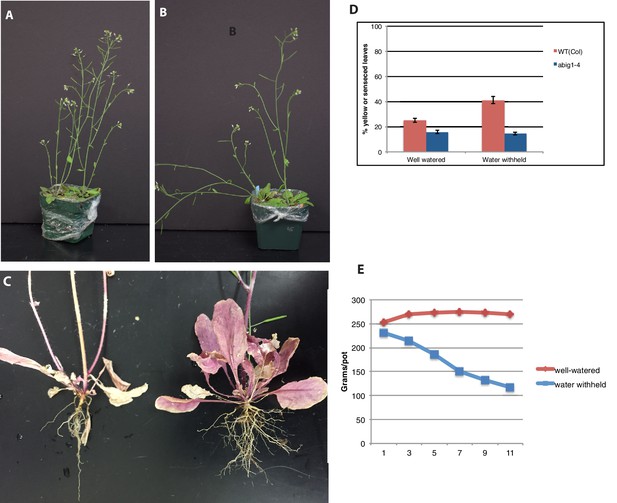
Response of abig1-4 mutants to drought treatment.
(A) Well watered plants. Wild-type Col-0 (left) and abig1-4 (right) plants grown in a shared pot. (B) Plants from which water was withheld for 17 days. Wild-type Col-0 (left) and abig1-4 (right) plants grown in a shared pot. (C) Root systems of wild type Col-0 (left) and abig1-4 (right) plants from which water was withheld. Plants were harvested at the end of their lifecycle at which point both genotypes are fully senesced. (D) Percentage of leaves that had turned yellow after 17 days of withheld water. (E) Rate of water loss from pots during the water-withholding period.
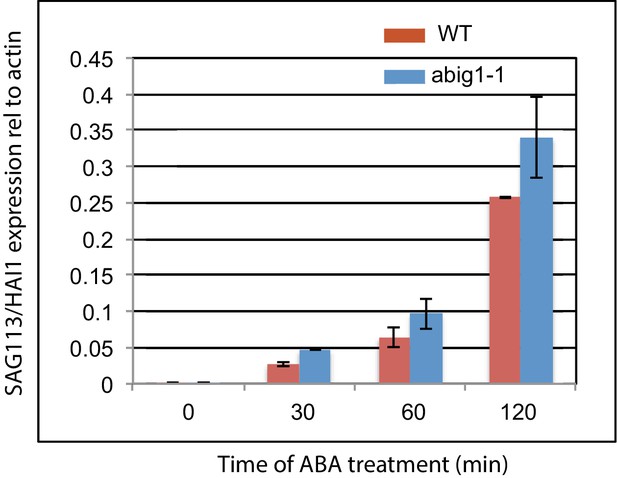
Measurement of levels of SAG113/HAI1 mRNA levels in wild type and abig1 mutants in response to exogenously added ABA.
An experiment was performed on three biological replicates, each with two technical replicates. ABA treatment was on 7 day old liquid grown seedlings treated with ABA for 120 min.

Response of abig1-1 mutant outcrossed to Columbia to drought.
(A) Well watered plants. Wild-type Col-0 (left) and abig1-1 (right) plants grown in a shared pot. (B) Plants from which water was withheld for 17 days. Wild-type Col-0 (left) and abig1-1 (right) plants grown in a shared pot.
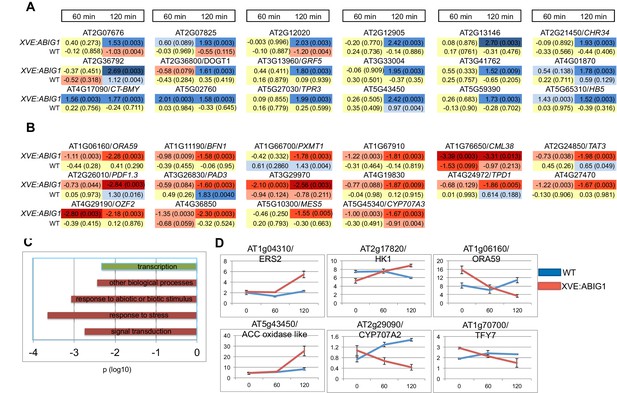
Genes regulated in response to ABIG1 induction by estradiol.
(A) Genes up-regulated in response to ABIG1 induction by estradiol. (B) Genes down-regulated in response to ABIG1 induction by estradiol. Fold change relative to time 0 is given in log base 2. Darker blue indicates increased fold change. Darker red indicates decreased fold change. The number in parenthesis is p value adjusted for multiple hypothesis testing. Time is given in minutes following estradiol addition. (C) Enrichment in gene ontology terms for genes up-regulated (green) and down-regulated (red) by induction of ABIG1 with estradiol. X axis indicates the level of significance (log 10 p) for each set of terms. (D) Expression of ethylene (left), ABA (middle) and jasmonate (right) pathway genes in response to ABIG1 induction with estrogen.
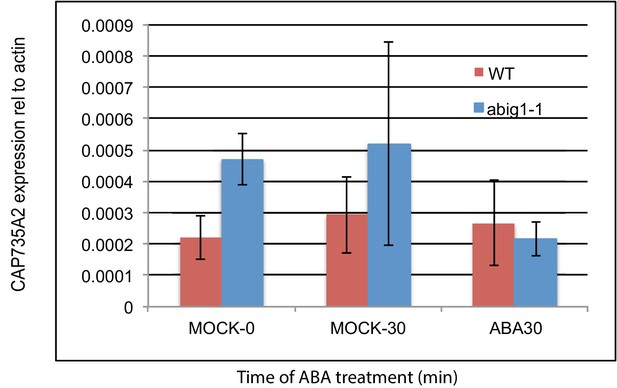
mRNA levels of cytokinin biosynthetic gene CYP735A2/At1g67110 in abig1 mutants and in response to exogenously added ABA.
Experiment shows the results of three biological replicates, each with three technical replicates. ABA treatment of liquid grown seven day old seedlings was for 30 min.
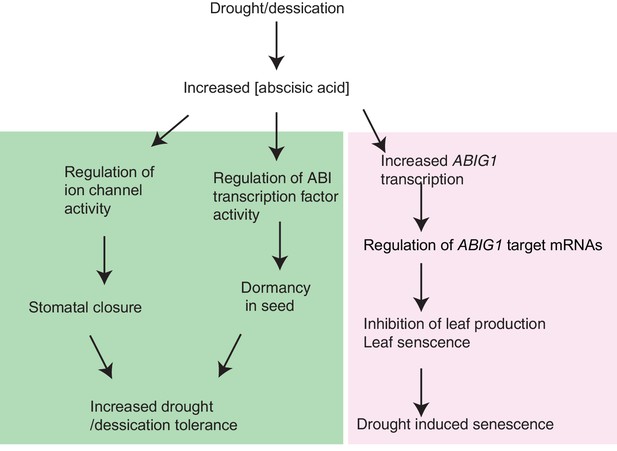
Model for ABIG1 action in the plant.
ABA concentration increases following drought. ABIG1 mRNA increases in response and, in a pathway that is distinct from the pathways controlling germination and stomatal closure, causes a reduction in growth and triggers early senescence. In the absence of ABIG1, plant growth is less affected by drought and leaf senescence is less likely to be triggered by drought.
Additional files
-
Supplementary file 1
RNA expression data tables.
(A) RNA sequence data for genes showing up-regulation in response to estradiol induction of XVE:ABIG1 plants. (B) RNA sequence data for genes showing down-regulation in response to estradiol induction of XVE:ABIG1 plants. (C) Expression of cytokinin network genes in response to estradiol induced activation of ABIG1. (D) Expression of ethylene network genes in response to estradiol induced activation of ABIG1. (E) Expression of abscisic acid network genes in response to estradiol induced activation of ABIG1. (F) Expression of chlorophyll degradation network genes in response to estradiol induced activation of ABIG1. (G) Expression of jasmonate network genes in response to estradiol induced activation of ABIG1. (H) Sequence of primers used for genotyping and for Q-RT-PCR.
- https://doi.org/10.7554/eLife.13768.015





