Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level
Peer review process
This article was accepted for publication as part of eLife's original publishing model.
History
- Version of Record published
- Accepted Manuscript published
- Accepted
- Received
Decision letter
-
Joaquín M EspinosaReviewing Editor; University of Colorado Denver School of Medicine, United States
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your work entitled "Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level" for consideration by eLife. Your article has been favorably evaluated by Jessica Tyler (Senior editor) and three reviewers, one of whom is a member of our Board of Reviewing Editors.
The reviewers have discussed the reviews with one another and the Reviewing Editor has drafted this decision to help you prepare a revised submission.
In the present manuscript, the authors report, using a nice experimental model, that activation of the TAp63 transcription factor leads to tetramerization that follows a spring-loaded mechanism in the absence of any translation of other cellular factors in oocytes and is associated with unfolding of the inhibitory structure that blocks the tetramerization interface. This is highly important, as the mechanism of activation of its more famous family member p53 is totally different. The consequences of this mechanism are extremely important to elucidate how quality control of oocytes is achieved. The data presented are of excellent quality and expand the area of TAp63 in the quality control of oocytes. Indeed, the detailed analysis of TAp63 structural analysis at the molecular level represents relevant new insight on reproductive biology as well as in understanding the mechanism of p63 regulation, as compared for example to p53. Consequently, this is a very important, highly interesting and solid study investigating the consequences of loss of p63, or its genetic mutations at the structural level.
However, the reviewers have a number of concerns about some of the data and its interpretation. Overall, the reviewers agreed to encourage resubmission of a revised manuscript addressing these concerns.
1) In Figure 6 the argument that p63 activation is post translationally regulated by phosphorylation is not substantiated by the data. The authors present an attractive model for activation of TAp63α upon DNA damage in oocytes. This is supported by demonstration of TAp63 tetramer formation and induction of p63 target gene expression (p21, Puma, Mdm2) in irradiated murine oocytes. However, this model is incomplete without data showing that TAp63 phosphorylation indeed triggers tetramerization and activation. The reviewers propose a number of experiments to address this issue. First, treatment of sample lysates with a phosphatase to show that the migration differences post-irradiation is indeed due to phosphorylation. It is possible that the differences in mobility are due to some other post-translational modification. Second, if, as they propose in their discussion, ATM or CHECK2 are putative culprits in activating TAp63, this needs to be demonstrated in some fashion, either via genetic or pharmacological inactivation of these kinases. This could also be tested in vitro by adding relevant kinases, i.e. ATM and/or CHEK2. In addition, specific phosphorylation sites in TAp63α could be mutated and the effect on tetramerization could be examined in vitro and in oocytes. This type of data would strengthen the manuscript considerably.
2) In the subsection “Defining the minimal sequence required for formation of the closed dimeric conformation”, the authors refer to three different constructs: TAp63αmin, wild type TAp63α, and a slightly shortened version TAp63α(10-614). Two constructs are shown in Figure 1 and Figure 1—figure supplement 2. It looks as if the slightly shortened version, TAp63α(10-614), is not shown. Yet the TAp63αmin construct contains exactly residues 10-614. This should be clarified. Which construct is shown?
3) In Figure 6, levels of TAp63 tetramers in irradiated oocytes are significantly lower than those of dimeric TAp63 prior to irradiation, probably less than 10%. This is apparent both on the native gel (B) and the denaturing SDS gel (C). Thus, it seems like the formation of tetramers is associated with a dramatic reduction in TAp63 protein levels. This is not discussed. What is the reason for the much lower levels of TAp63 tetramers? p63 target genes are induced in irradiated oocytes (Figure 6). Are p53 and/or p73 expressed in these cells upon DNA damage? Did the authors exclude the possibility that the observed induction of target genes is due to activation of p53 or p73?
4) It would be important to measure caspase activation in response to ectopic p63 expression in SAOS cells using the p63 mutants.
https://doi.org/10.7554/eLife.13909.032Author response
1) In Figure 6 the argument that p63 activation is post translationally regulated by phosphorylation is not substantiated by the data. The authors present an attractive model for activation of TAp63α
upon DNA damage in oocytes. This is supported by demonstration of TAp63 tetramer formation and induction of p63 target gene expression (p21, Puma, Mdm2) in irradiated murine oocytes. However, this model is incomplete without data showing that TAp63 phosphorylation indeed triggers tetramerization and activation. The reviewers propose a number of experiments to address this issue. First, treatment of sample lysates with a phosphatase to show that the migration differences post-irradiation is indeed due to phosphorylation. It is possible that the differences in mobility are due to some other post-translational modification. Second, if, as they propose in their discussion, ATM or CHECK2 are putative culprits in activating TAp63, this needs to be demonstrated in some fashion, either via genetic or pharmacological inactivation of these kinases. This could also be tested in vitro by adding relevant kinases, i.e. ATM and/or CHEK2. In addition, specific phosphorylation sites in TAp63α could be mutated and the effect on tetramerization could be examined in vitro and in oocytes. This type of data would strengthen the manuscript considerably.
The reviewers wanted to see a direct link between phosphorylation and activation and asked to provide evidence that the shift seen on the SDS gel of TAp63α following irradiation is due to phosphorylation. We had provided such evidence already in the Cell paper (Deutsch et al., 2011). There we have treated oocyte lysates after irradiation showing both tetramerization as well as a shift on an SDS gel with λ-phosphatase. Despite the complete removal of the shift on the SDS gel the gel filtration profile of the now non-phosphorylated TAp63α remains virtually the same, indicating that 1) the shift is indeed due to phosphorylation and 2) that phosphorylation is only the trigger to induce the change from a dimeric to a tetrameric state, supporting our analysis that the dimeric state is a high energy kinetically trapped one. To make this more clear we have added the citation of our Cell paper to the section in which we have mentioned these experiments in the original version of the manuscript (“The dimeric conformation of TAp63α constitutes a kinetically trapped state”) and have added a few sentences in the Discussion part.
In addition, the reviewers asked us to prove that phosphorylation triggers activation and tetramerization. Activation through phosphorylation by Chk2 has been shown in the paper by Bolcun-Filas et al. (Science 2014) that we cite several times. They had, however, not linked activation to tetramerization. To make this link we have added experimental results to Figure 6 that show that addition of 25 µM of a Chk2 inhibitor results in the suppression of phosphorylation and tetramerization. Furthermore, these experiments demonstrate that inhibiting the activation of TAp63α also results in stabilization of the protein. This is consistent with earlier investigations that had shown a link between proteasomal degradation and active DNA binding and transactivation. The observation that inhibition of activation results in higher cellular protein levels of TAp63α also supports our model that in the inhibited state the TAD is not accessible and only the open state can be degraded fast. We discuss all of this in a new paragraph at the end of the Results section.
2) In the subsection “Defining the minimal sequence required for formation of the closed dimeric conformation”, the authors refer to three different constructs: TAp63αmin, wild type TAp63α, and a slightly shortened version TAp63α(10-614). Two constructs are shown in Figure 1 and Figure 1—figure supplement 2. It looks as if the slightly shortened version, TAp63α(10-614), is not shown. Yet the TAp63αmin construct contains exactly residues 10-614. This should be clarified. Which construct is shown?
The reviewers asked to clarify the exact boundaries of the different constructs used. We have modified Figure 1—figure supplement 2 now showing all three different versions of TAp63α used in the experiments reported in this manuscript.
3) In Figure 6, levels of TAp63 tetramers in irradiated oocytes are significantly lower than those of dimeric TAp63 prior to irradiation, probably less than 10%. This is apparent both on the native gel (B) and the denaturing SDS gel (C). Thus, it seems like the formation of tetramers is associated with a dramatic reduction in TAp63 protein levels. This is not discussed. What is the reason for the much lower levels of TAp63 tetramers? p63 target genes are induced in irradiated oocytes (Figure 6). Are p53 and/or p73 expressed in these cells upon DNA damage? Did the authors exclude the possibility that the observed induction of target genes is due to activation of p53 or p73? The reviewers asked about the reduced cellular levels of TAp63α following activation. As explained above this is due to fast proteasomal degradation of active p63 (see response to question #1). We have also investigated if p53 or p73 can be detected before or after irradiation in oocytes. Using immunohistochemistry, we have not been able to detect p53 and only a weak and diffuse staining for p73. The much higher concentration of p63 together with the fact that significant induction of target genes is achieved in the presence of the translation inhibitor cycloheximide (preventing an increase of the cellular content of p73 through transcription by p63) strongly argues that the transcriptional effects seen are due to p63. We describe these results in the last paragraph of the Results section and provide the data in Figure 6—figure supplement 1.
4) It would be important to measure caspase activation in response to ectopic p63 expression in SAOS cells using the p63 mutants.
We have tried to show caspase activation in response to ectopic expression of activated p63 mutants in different cell lines (SAOS, H1299, SUM159) but were unable to detect apoptosis in these cell lines (no cleaved PARP or activated caspase-3). This might be due to effective mechanisms of these cancer cell lines to suppress apoptosis or that additionally DNA damage is necessary to activate the apoptotic program.
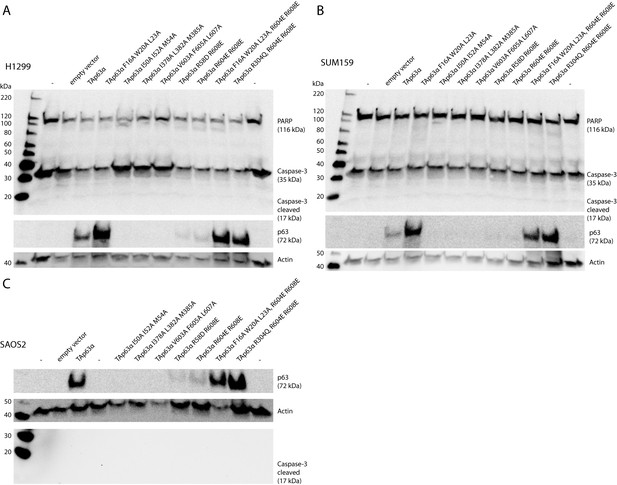
Caspase assay.
H1299 (A), SUM159 (B) and SAOS2 (C) cells were transfected with TAp63α mutants. Caspase-3 Antibody (Cell Signaling #9662) and PARP Antibody (Cell Signaling #9542) were used in A and B to detect full-length proteins and cleaved fragments (large fragment of caspase-3 (17 kDa) and large fragment of PARP (89 kDa)). Cleaved Caspase-3 Antibody (Cell Signaling #9662) was used in C to detect cleaved Caspase-3. No signal of any cleaved fragment was detected in dependency of TAp63α mutant transfection. Only inactive TAp63α (either wildtype or mutants bearing mutation F16A W20A L23A or R304Q (DNA-binding deficient)) reached high expression levels in the cells.





