Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate
Figures
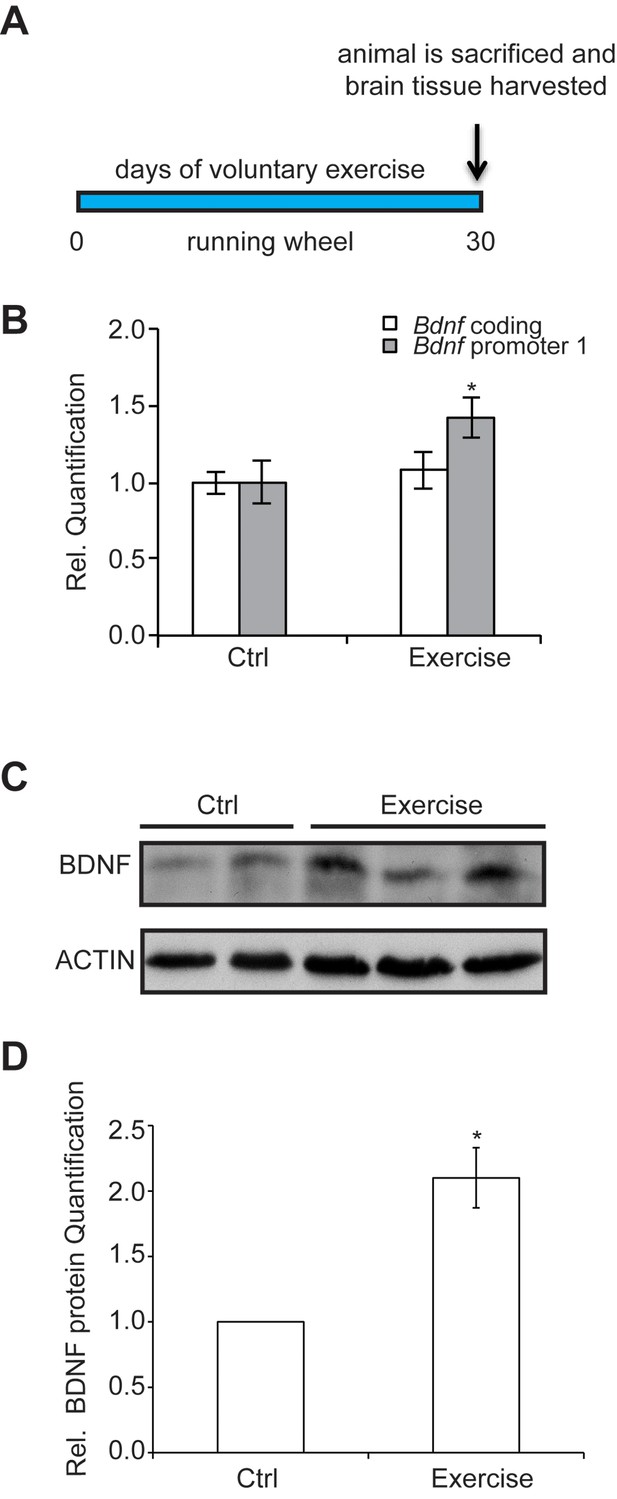
The experimental design and time course of the exercise regime is shown.
Exercise induces changes in brain BDNF levels in a voluntary exercise protocol (16). (A) Experimental design for the Voluntary exercise model. (B) Voluntary exercise for 4 weeks significantly induces Bdnf promoter I expression in the hippocampus as measured by real-time RTPCR. The number of animal used for each group (control and exercise) is 10. *p<0.05 as measured by unpaired t-test. (C) Western blot analysis depicting the increase in mature BDNF protein levels in the hippocampus of exercise animals as compare to wild type. In this representative image, the BDNF levels from 2 control hippocampal lysates and 3 exercise hippocampal lysates are depicted. This experiment was replicated from additional 3 different animals in each group. (D) Quantification of the BDNF western blot.
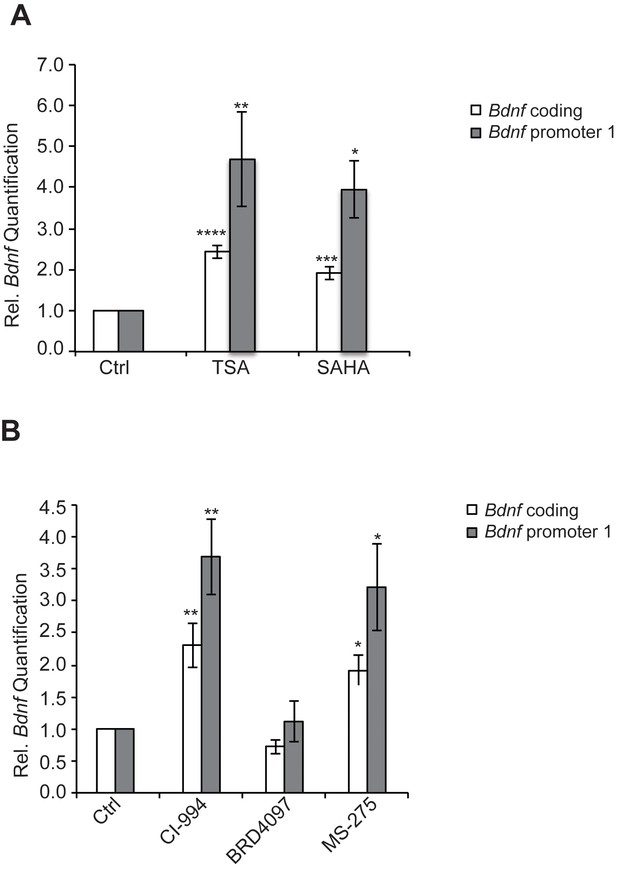
HDAC inhibitors induce Bdnf expression.
(A) Broad spectrum HDAC inhibitors such as TSA (0.67 μM) and SAHA (5 μM) induce coding and pI Bdnf expression levels as measured by real-time RTPCR. For the coding promoter, the n number for controls, TSA and SAHA treatments are 6, 5 and 4 respectively. For the pI promoter, the n number for controls, TSA and SAHA treatments are 5, 5 and 4 respectively. Each replicate consisted of primary neurons obtained from different set cultures and treated with fresh dilutions of the compounds. Significance was measured by 1way anova **p< 0.01.and ***p<0.001. (B) Treatment with class I HDAC inhibitors such as CI-994 (10 μM) and MS-275 (10 μM) induce coding and pI Bdnf levels, whereas the negative control analog for CI-994, BRD4097 does not as measured by real-time RTPCR. For the coding promoter, the n number for controls, CI-994, BRD4097 and MS-275 treatments are 6, 6, 5 and 5 respectively. For the pI promoter, the n number for controls, CI-994, BRD4097 and MS-275 are 5, 6, 5 and 5 respectively. Each replicate consisted of primary neurons obtained from different cultures and treated with fresh dilutions of the compounds. Significance was measured by 1way anova **p<0.01.and ***p<0.001.
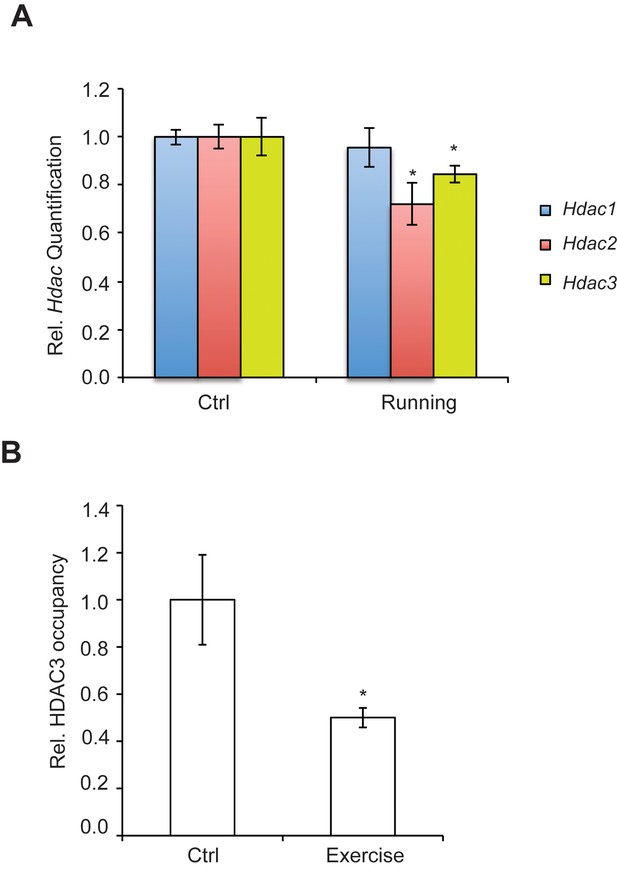
Exercise affects Hdac expression and binding to the hippocampal BDNF promoter.
(A) Exercise induces significant decreases in both Hdac2 and Hdac3 expression in the hippocampus without affecting Hdac1 expression as measured by real-time RTPCR. The expression was analyzed from the hippocampi of 4 different control and exercise animals. Unpaired t-tests were used to measure statistical significance *p<0.05. (B) Exercise induces decreases in HDAC3 binding to the Bdnf promoter as measured by chromatin immunoprecipitation followed by real-time RTPCR. The experiment was conducted by using tissues from 7 different control and exercise animals. Unpaired t-tests were used to measure statistical significance *p<0.05.
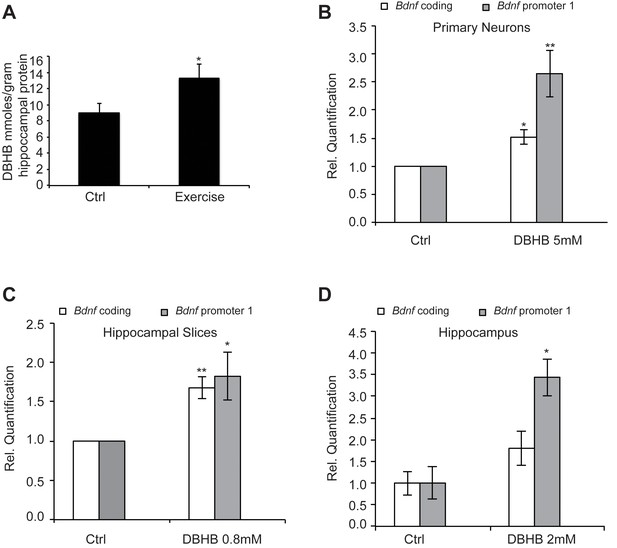
Exercise increases DBHB levels in the hippocampus.
DBHB in turn can induce Bdnf expression in vitro in neurons and in vivo in the hippocampus (A) Exercise induced DBHB levels in the hippocampus. The number for controls and exercise hippocampi is 10 and 11 respectively. Statistical significance was analyzed by the unpaired t-test *p<0.05. DBHB amounts are expressed as millimole per gram of total hippocampal protein (B) Overnight treatment of DIV6 primary cortical neurons with 5 mM of DBHB significantly induces coding and pI Bdnf expression levels as measured by real-time RTPCR. For both promoters, the n number for controls and DBHB are 4 and 4 respectively. Each replicate consisted of primary neurons obtained from different cultures and treated with fresh dilutions of the compounds. Significance was measured by unpaired t-test *p<0.05 and **p<0.01. (C) Three hour treatment of hippocampal slices with DBHB (0.8 mM) induces coding and pI Bdnf levels as measured by real-time RTPCR. For both promoters, the n number for controls and DBHB are 5 and 5 respectively. Each replicate consisted of slices obtained from different animals and treated with fresh dilutions of the compounds. Significance was measured by unpaired t-test *p<0.05 and **p<0.01. (D) Intraventricular delivery of DBHB (2 mM) significantly induces coding and pI Bdnf levels as measured by real-time RTPCR. The n number for controls and DBHB are 3 and 5 respectively. Each replicate consisted of hippocampi from different animals that were subjected to the surgical procedure. Significance was measured by unpaired t-test *p<0.05.
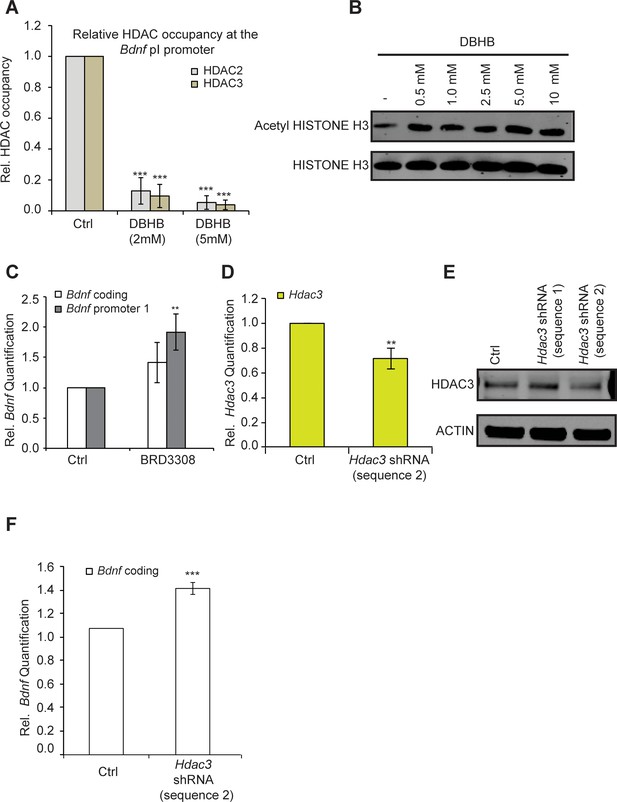
DBHB induces Bdnf expression by inhibiting HDAC2 and HDAC3.
(A) DBHB (2 mM or 5 mM) treatment decreased the binding of both HDAC2 and HDAC3 on the pI promoter of Bdnf as measured by chromatin immunoprecipitation followed by real-time RTPCR. The number of chromatin immunoprecipitations for each HDAC for each treatment were 3 (control), 3 (DBHB 2 mM) and 2 (DBHB 5 mM). Statistical significance was measured by 1way anova ****p<0.001. (B) HISTONE H3 acetylation is increased in neurons upon treatment with different doses of DBHB. Representative blot shown in the figure. (C) The HDAC3 selective inhibitor BRD3308 significantly induces Bdnf pI expression as measured by real-time RTPCR.. For the coding promoter, the n number for controls and BRD3308 treatments are 6 and 3 respectively. For the pI promoter, the n number for controls and BRD3308 treatments are 5 and 4 respectively. Each replicate consisted of primary neurons obtained from different cultures and treated with fresh dilutions of the compounds. Significance was measured by unpaired t-test **p<0.01. (D) Hdac3 knockdown is verified by real-time RTPCR. N = 5 represents independent times knockdown was achieved in different primary culture using nucleofection. Significance was measured by unpaired t-test **p<0.01. (E): HDAC3 knockdown is verified by western blotting. (F): HDAC3 knockdown significantly induces Bdnf coding gene expression as verified by real-time RTPCR. N = 5 and significance was measured by unpaired t-test *p<0.05. The Hdac3 shRNA sequence 2 that significantly reduced HDAC3 protein levels induced Bdnf expression.
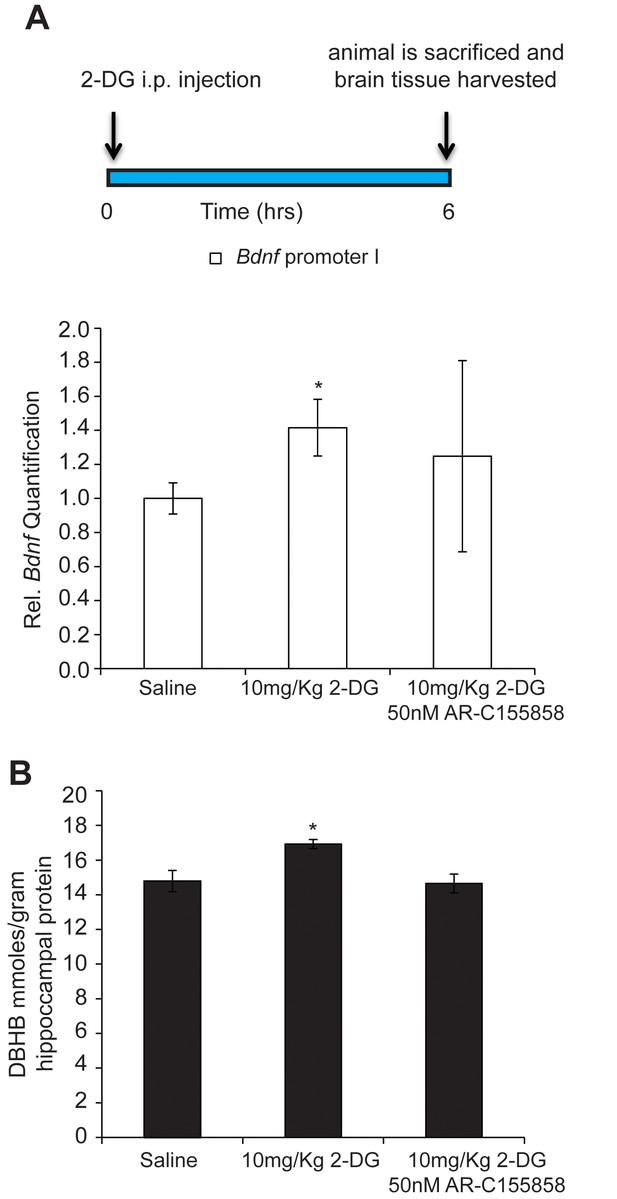
DBHB can serve as an exercise factor linking metabolic changes in response to exercise to changes in gene expression in the brain.
(A) 2-DG treatment known to induce ketone bodies in the brain induces Bdnf pI expression in the hippocampus as measured by real-time RTPCR. This effect was blocked by a DBHB transporter inhibitor. N = 10 (Ctrl), N = 9 (2-DG injected) and N = 5 (2-DG and AR-C155858). Significance was measured by unpaired t-test *p<0.05. (B) 2-DG treatment induced DBHB levels in the hippocampus and this effect is blocked by a DBHB transporter inhibitor.
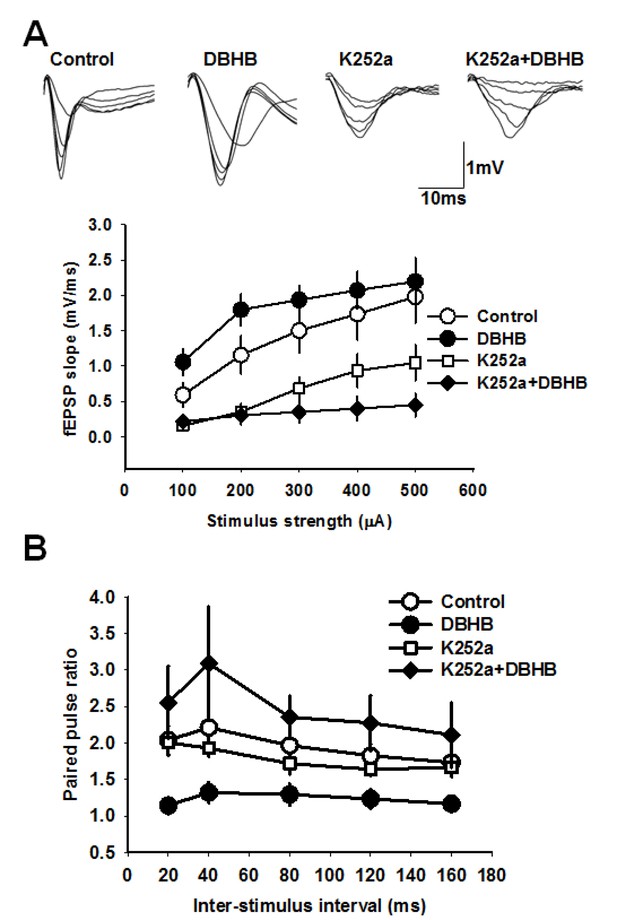
DBHB increases glutamatergic transmission at the CA3-CA1 synapses in a TrkB-sensitive manner.
(A) Average fEPSP slope in control (6 slices/6 mice), DBHB (6 slices/6 mice), K252a (6 slices/3 mice), and K252a+DBHB (6 slices/3 mice) groups. Two-way repeated measures ANOVA showed significant difference between groups (F3,20 = 10.13, p<0.001). Upper panel shows examples fEPSP traces. (B) Average paired pulse ratio in control (6 slices/6 mice), DBHB (6 slices/6 mice), K252a (6 slices/3 mice), and K252a+DBHB (6 slices/3 mice) groups. Two-way repeated measures ANOVA showed significant difference between groups (F3,20 = 4.03, p = 0.022).
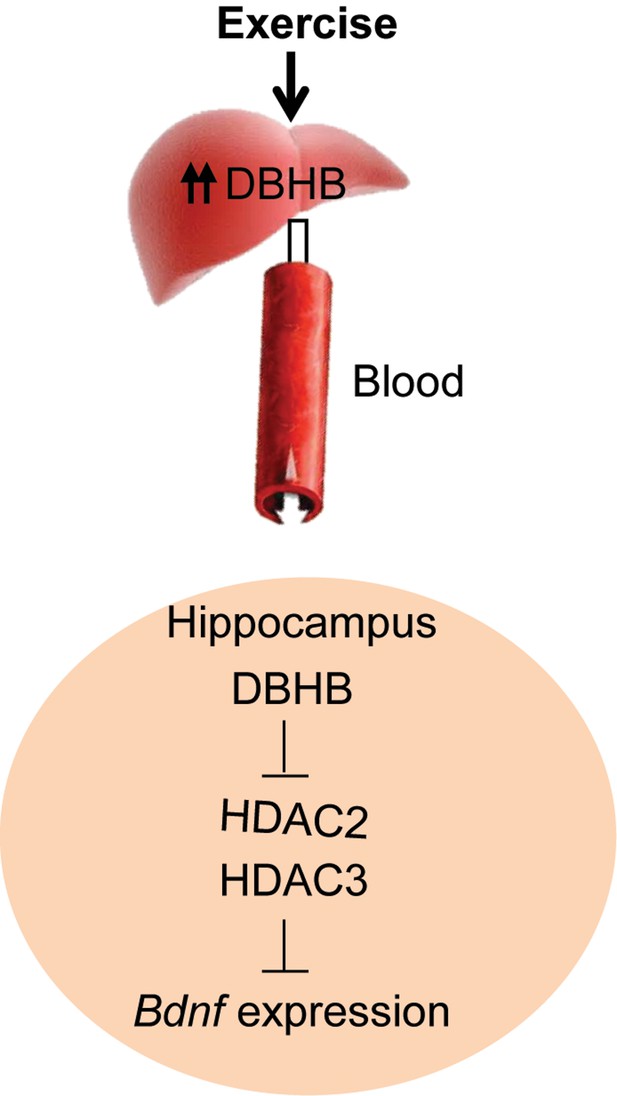
A proposed model by which exercise induces Bdnf expression in the hippocampus.
Exercise induces DBHB synthesis in the liver. DBHB is transported through the circulation to peripheral organ including the brain. In the hippocampus, DBHB induces Bdnf expression through a mechanism involving HDAC inhibition. This induction in turn mediates exercise’s positive effects on memory, cognition and synaptic transmission.





