Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis
Figures
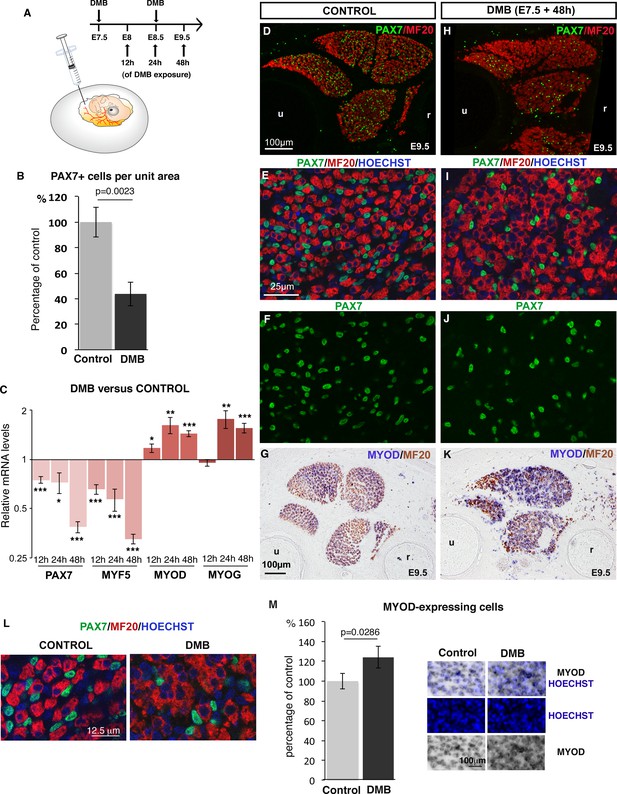
Inhibition of muscle contraction decreases the number of limb fetal muscle progenitors.
(A) Chick fetuses were treated with DMB at E7.5 and E8.5, in order to block muscle contraction. (B) Number of PAX7+ cells in paralyzed and control muscles. PAX7+ cell number was counted per unit area in dorsal and ventral muscles of three DMB limbs and three control limbs. Results are shown as percentage of control. Error bars indicate standard deviations. The p-value was obtained using the Mann-Withney test. (C) RT-q-PCR analyses of muscle gene expression levels in limbs 12 hr, 24 hr and 48 hr after DMB treatment compared to control limbs. For each gene, the mRNA levels of control limbs were normalized to 1. Graph shows means ± standard errors of the mean of 11 limbs. The p-values were calculated using the Wilcoxon test. Asterisks indicate the p-values *p<0.05, **p<0.01, ***p<0.001. (D–K) Control limbs (D–G) (N = 5) and limbs from DMB-treated embryos (H–K) (N = 5) were transversely sectioned at the level of the zeugopod and analyzed for muscle progenitors and differentiated cells by immunohistochemistry using the PAX7 and MF20 antibodies, respectively (D–F, H–J), or for MYOD expression by in situ hybridization followed by immunohistochemistry with MF20 antibody (G,K) (N = 4). Nuclei were labeled with Hoechst (blue). Limb sections are dorsal to the top and posterior to the left. u, ulna, r, radius. (L) High magnifications of muscle fibers to show the grouped nuclei in fibers of paralyzed muscles compared to control muscles. (M) Number of Hoechst+ nuclei of MYOD-expressing cells versus all Hoechst+ nuclei. Results are shown as percentage of control. Error bars indicate standard deviations. The p-value was obtained using the Mann-Withney test.
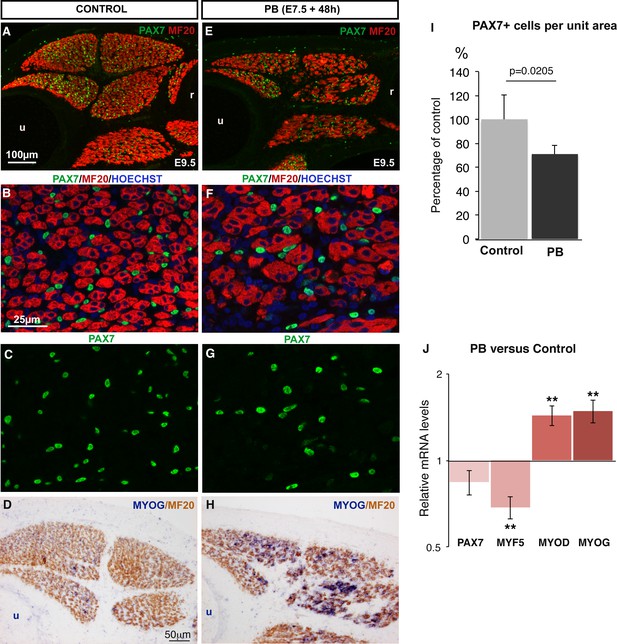
Muscle flaccid paralysis decreases the number of PAX7+ muscle progenitors and increases their differentiation.
Chick embryos were treated with Pancuronium bromide (PB) at E7.5 and E8.5 to induce muscle flaccid paralysis. Embryos were processed 48 hr after PB exposure (at E9.5). Control (A–D) and PB-treated (E–H) limbs were transversely sectioned and analyzed by immunohistochemistry (A–C,E–G) (N = 3) or by in situ hybridization followed by immunohistochemistry (D,H) (N = 3). Immobilized muscles visualized with MF20 were decreased in size compared to control muscles (E versus A). PB-treated embryos displayed a diminution in the number of PAX7+ cells in limb muscles (F,G) compared to limb muscles of control embryos (B,C). (D,H) MYOG expression was increased in immobilized (H) versus control (D) muscles. (I) Number of PAX7+ cells in paralyzed and control muscles. PAX7+ cell number was counted per unit area in dorsal and ventral muscles of three PB limbs and three control limbs. Results are shown as percentage of controls. Error bars indicate standard deviations. The p-value was calculated using the non-parametric Mann-Whitney test. (J) RT-q-PCR analyses of muscle gene expression levels in limbs 48 hr after PB treatment compared to control limbs. For each gene, the mRNA levels of control limbs were normalized to 1. The p-values were calculated using the non-parametric Wilcoxon test. Error bars indicate standard errors of the mean of eleven samples. Asterisks indicate the p-values **p<0.01. u, ulna, r, radius.
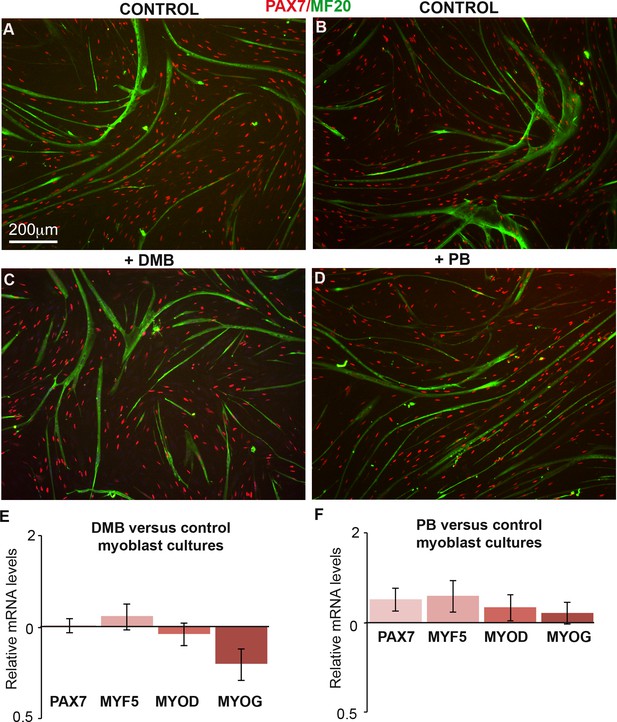
DMB or PB treatment in primary cultures of chick fetal myoblasts did not change the expression of muscle genes.
(A–D) Primary cultures of chick fetal myoblasts were treated with DMB or PB for 48 hr and fixed for immunochemistry. DMB-treated (C) or PB-treated (D) myoblasts displayed similar PAX7 and MF20 staining compared to control cultures (A,B). (E,F) RT-q-PCR analyses of muscle gene expression levels in cultured fetal myoblasts 48 hr after DMB (E) (N = 6) or PB (F) (N = 6) exposure compared to control cultures (N = 6). For each gene, the mRNA levels of control cultures were normalized to 1. Graphs show the means ± standard errors of the mean. The relative mRNA levels of PAX7, MYF5, MYOD and MYOG genes were not significantly changed in fetal myoblasts treated with DMB or PB compared to controls.
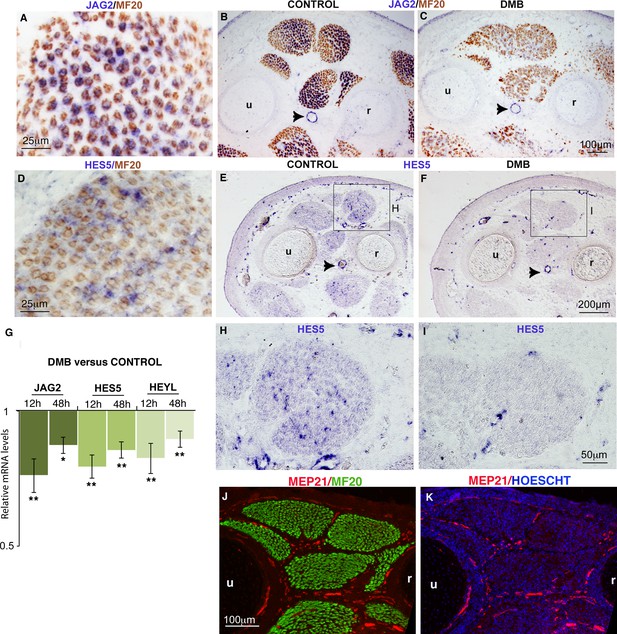
Muscle contraction inhibition decreases NOTCH activity in limb muscles.
(A–F, H–I) In situ hybridization to transverse limb sections at the level of the zeugopod of E9.5 chick fetuses treated (C,F,I) or not treated (A,B,D,E,H) with DMB, with JAG2 (A–C) or HES5 (D–F,H,I) probe followed by immunostaining with MF20 antibody (A–D) (N = 4). The expression of the NOTCH ligand JAG2 was lost in muscle fibers of paralyzed muscles (C) compared to JAG2 normal expression (A,B). (B,C, arrowheads) JAG2 expression in blood vessels was not affected in DMB limbs. The expression of the transcriptional readout of NOTCH, HES5 was also decreased in paralyzed muscles (F,I) compared to control muscles (D,E,H). (G) RT-q- PCR analyses of the expression levels of NOTCH signaling components in limbs of 12 hr and 48 hr DMB-treated fetuses. For each gene, the mRNA levels of control limbs were normalized to 1. Graph shows means ± standard errors of the mean of eight limbs. The p-values were calculated using the Wilcoxon test. Asterisks indicate the p-values, *p<0.05, **p<0.01. (J,K) Transverse limb sections of E9.5 fetuses were immunostained with MEP21 and MF20 antibodies to visualize blood vessels and differentiated muscles, respectively (N = 3). Hoescht was used to visualize nuclei in blue. Limb sections are dorsal to the top and posterior to the left. u, ulna, r, radius.
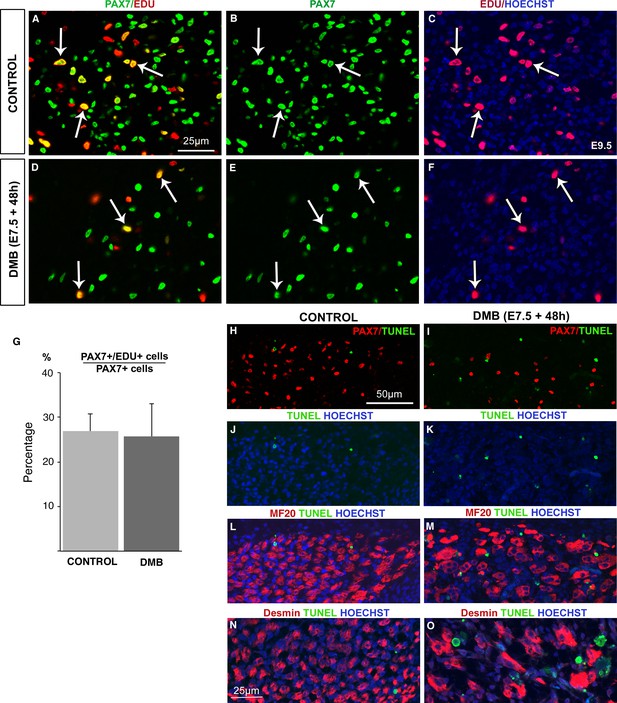
The proliferation rate of muscle progenitors is not modified in paralyzed muscles.
Limb muscles of control (A–C) and DMB-treated (D–F) fetuses were analyzed for cell proliferation by EdU incorporation. Control and paralyzed muscles displayed EdU+/PAX7+ cells showing proliferating muscle progenitors (A–F, arrows). The percentage of EdU+/PAX7+ cells on PAX7+ cells was analyzed in three muscles of three DMB limbs and three control limbs. (G) The percentage of EdU+/PAX7+ cells on PAX7+ cells was similar in both control and paralyzed muscles. Error bars show standard deviations. Control (H,J,L,N) and paralyzed (I,K,M,O) muscles were analyzed for apoptosis. TUNEL staining was rarely visualized in control muscles (H,J,L,N), while paralyzed muscles displayed an increase of apoptotic figures (I,K,M,O), which were not located in PAX7+ cells (I), in Myosin+ cells (M) or in Desmin+ cells (O).
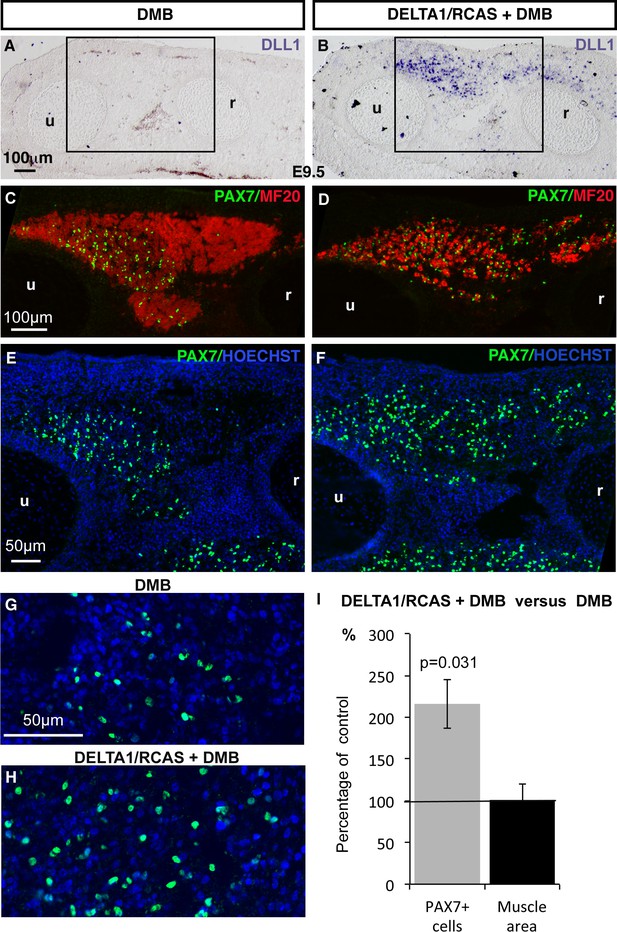
Ligand-induced NOTCH activity prevents the diminution in the number of fetal muscle progenitors and the increase of muscle differentiation in immobilized fetuses.
Transverse sections of contralateral (A,C,E,G) and DELTA1/RCAS grafted (B,D,F,H) limbs of DMB-treated fetuses were hybridized with the DLL1 probe (A,B) to visualize ectopic DLL1 expression in right grafted limbs (B) or immunostained with PAX7 and MF20 antibodies (C–H) (N = 3). (I) The quantification of PAX7+ cell number per unit area and muscle area was performed in DELTA1/RCAS-grafted and contralateral limbs of the same immobilized embryos. Result was presented as percentage of contralateral limbs, in immobilization conditions. Error bars represent standard deviations of six sections originating from three independent experimental embryos. The p-value was calculated using the Wilcoxon test. Limb sections are dorsal to the top and posterior to the left. u, ulna, r, radius.
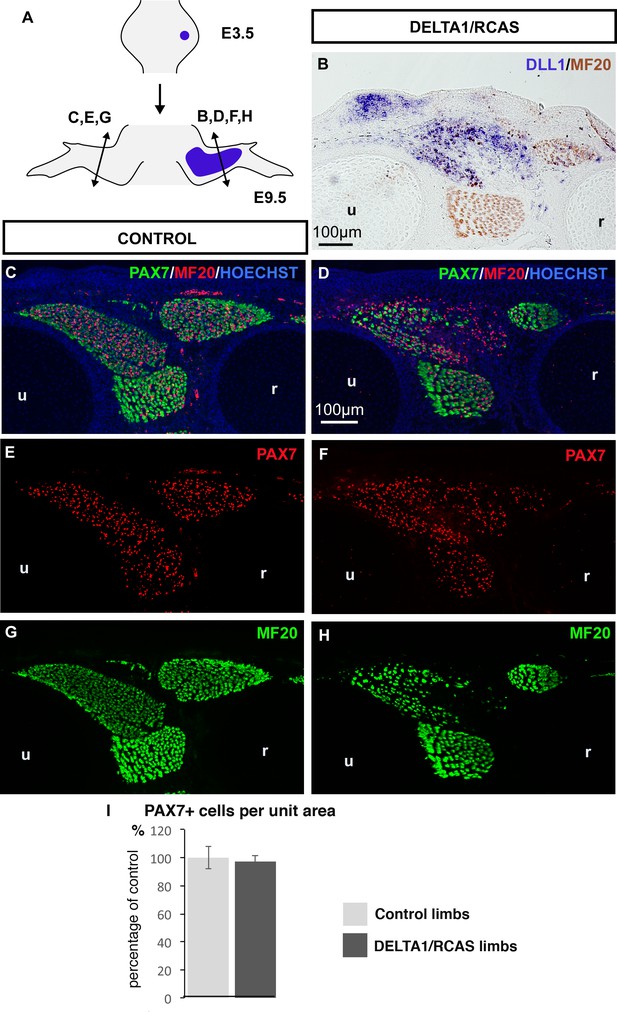
A continuous source of NOTCH ligand maintained the number of PAX7+ muscle progenitors despite the inhibition of muscle differentiation.
(A) DELTA1/RCAS-producing cells were grafted into limb buds of E3.5 chick embryos. Grafted-embryos were fixed 6 days later at E9.5 (N = 3). DELTA1/RCAS-grafted (B,D,F,H) and contralateral (C,E,G) limbs were cut transversely and analyzed for DLL1 expression by in situ hybridization (B) or for muscle progenitors and differentiated cells by immunohistochemistry using PAX7 and MF20 antibodies (C–H). Hoechst was used to visualize nuclei. Ectopic DLL1 expression visualized in dorsal muscle masses (B) maintained the pool of PAX7+ cells (D,F) compared to contralateral limbs (C,E) despite the decreased number of muscle fibers (H versus G). (I) PAX7+ cell number was counted per unit area in DLL1-infected-muscles of three DELTA1/RCAS-grafted and in equivalent muscles in control limbs. Results are shown as percentage of controls. Error bars indicate standard deviations. Sections are dorsal to the top and posterior to the left. u, ulna, r, radius.
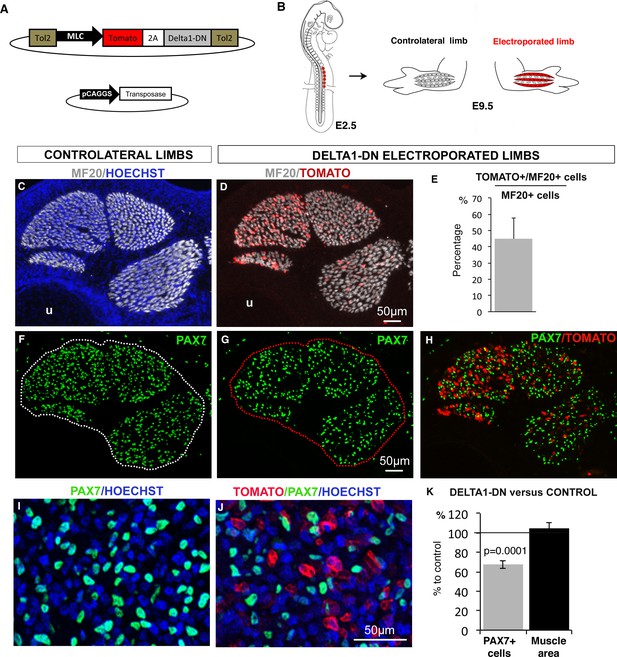
NOTCH-ligand activity in differentiated muscle cells is required to maintain the pool of fetal muscle progenitors.
(A) Schematic representation of the recombinant vector co-expressing the Tomato reporter gene and a dominant-negative form of DELTA1 (DELTA1/DN) under the control of the Myosin Light Chain (MLC) promoter between two Tol2 transposons, and of the transient vector containing the transposase. (B) E2.5 chick embryos were electroporated at the level of forelimb somites in order to target limb muscle cells. Electroporated and contralateral limbs from the same animals (N = 4) were analyzed 7 days after electroporation, at E9.5. (C,D, F–J) Transverse sections of electroporated and contralateral limbs were immunostained with PAX7 and MF20 antibodies and labeled with Hoechst to visualize nuclei in blue. (D,E) In electroporated muscles displaying Tomato expression (D), an average of 44.94% (±12.88) of MF20+ cells displayed Tomato expression (E). The size and shape of the electroporated muscles was not affected (D) compared to control muscles (C). Electroporated muscles displayed a decrease in the number of PAX7+ cells (G,H,J) compared to contralateral limbs (F,I). (K) The number of PAX7+ cells per unit area and the muscle area were analyzed in electroporated muscles and equivalent muscles of the contralateral limbs (originating from the same experimental animals). Results were presented as percentage of the contralateral limbs (control). The graph shows means ± standard errors of the mean of 14 sections originating from four electroporated embryos. The p-value was calculated using the Wilcoxon test. Limb sections are dorsal to the top and posterior to the left. u, ulna.
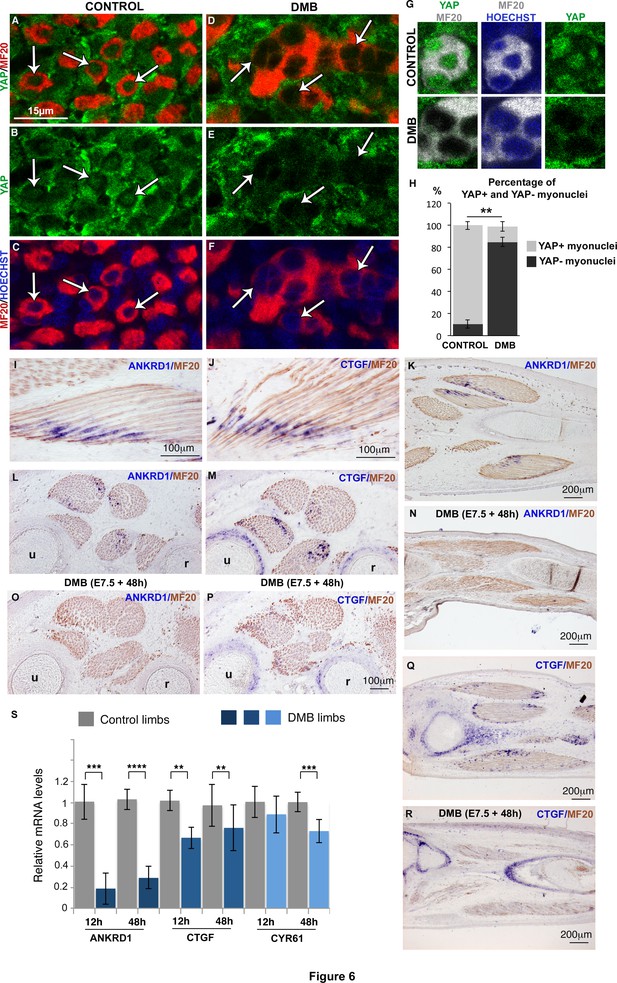
YAP activity is observed in contracting muscle fibers and lost in paralyzed muscles.
(A–F) Transverse limb muscle sections of E9.5 control (A–C) and DMB-treated fetuses (D–F) were immunostained with YAP and MF20 antibodies and labeled with Hoechst to visualize nuclei in blue (N = 3). (A–C) In myofibers, YAP was preferentially localized in myonuclei (arrows). (D–F) In paralyzed limb muscles, the nuclear localization of YAP protein in muscle fibers (MF20+ cells in red) was lost (D–F, arrows) compared to control muscles (A–C, arrows). (G) Focus on YAP+ myonuclei in MF20+ cells in control muscles and YAP- myonuclei in paralyzed muscles (DMB). (H) Quantification of the percentage of YAP+ myonuclei in MF20+ cells in control and paralyzed muscles. In situ hybridization to YAP target genes, ANKRD1 (I,K,L,N,O) and CTGF (J,M,Q,R) followed by immunohistochemistry with MF20 antibody in control limbs (I-M, Q) and paralyzed muscles (N,O,P,R) (N = 4). (I–M, Q) The YAP target genes were expressed at the tips of muscle fibers (blue staining in MF20+ cells in brown) visualized on longitudinal (I–K,Q) and transverse (L,M) muscle sections. ANKRD1 was exclusively expressed in limb muscles (L,K), while CTGF (M,Q) displayed additional expression in cartilage. (N,O,P,R) In the absence of muscle contraction, the expression of ANKRD1 and CTGF was lost in muscles but not in cartilage for CTGF. u, ulna, r, radius. For transverse limb sections, dorsal is to the top and posterior to the left. For longitudinal sections, dorsal is to the top and proximal to the left. (S) RT-q-PCR analyses of the expression levels of YAP target genes in limbs of 12 hr and 48 hr DMB-treated embryos. For each gene, the mRNA levels of control limbs were normalized to 1. The graph shows means ± standard errors of the mean of nine biological replicates. The p-values were calculated using the Mann-Whitney test. Asterisks indicate the p-value, **p<0.01, ***p<0.001, ****p<0.0001.
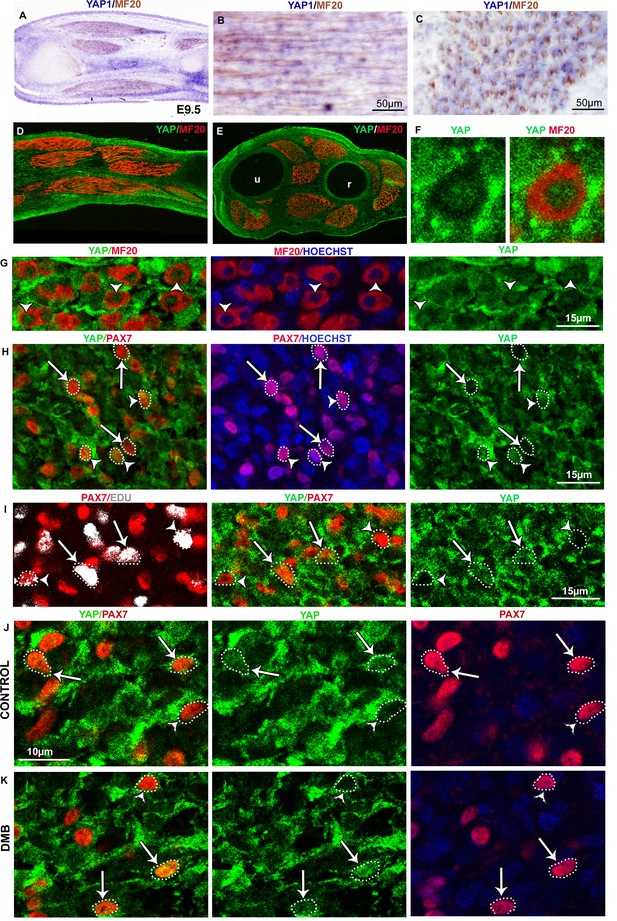
YAP expression and activity in limb muscles of control and immobilized fetuses.
Limbs from E9.5 chick fetuses were longitudinally (A,B,D) or transversally (C,E–G) sectioned and analyzed for YAP1 expression by in situ hybridization (blue) followed by immunochemistry with the MF20 antibody to visualize myosins (brown) (A–C) or for YAP protein expression (green) by double immunohistochemistry with MF20 antibody (red) (D–G). Hoechst was used to visualize nuclei. YAP1 transcripts and YAP protein were ubiquitously expressed in limbs. (F,G) High magnifications of muscle transverse sections showed YAP protein in myonuclei of postmitotic MF20+ muscle fibers (G, arrowheads). (F) YAP staining was higher in myonuclei compared to cytoplasms in MF20+ cells. (H) Muscle transverse sections co-immunostained with PAX7 (red) and YAP (green) showed that PAX7+ cells could be either nuclear YAP+ (arrowheads) or nuclear YAP− (arrows). (I) Chick limbs from E9.5 embryos treated with EdU were analyzed by immunohistochemistry for PAX7, YAP and EdU. Transverse limb sections co-immunostained with PAX7 (red), YAP (green) and EdU (grey) antibodies showed that the PAX7+/EdU+ cells were either nuclear YAP+ (I, arrows) or nuclear YAP- (I, arrowheads). (J,K) Transverse limb sections of control (J) or DMB-treated (K) E9.5 embryos were immunostained with YAP and PAX7 antibodies. PAX7+ cells displaying nuclear YAP staining (J, arrows) or no nuclear YAP staining (J, arrowheads) were observed in control and paralyzed muscles (J,K, arrows and arrowheads). u, ulna, r, radius.
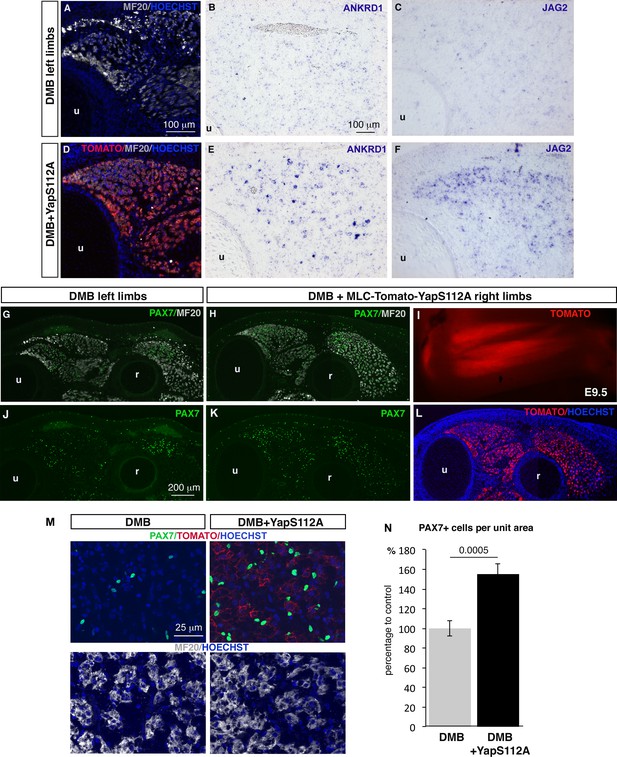
Forced YAP activity in differentiated muscle fibers prevents the decrease of ANKRD1 and JAG2 expression and in the number of fetal muscle progenitors in immobilized fetuses.
E2.5 chick embryos were electroporated at the level of forelimb somites with MLC-Tomato-YapS112A to force YAP activity in differentiated muscle cells, and then treated with DMB. Contralateral and electroporated limbs from the same immobilized animals (N = 4) were analyzed 7 days after electroporation, at E9.5. (A–F) Adjacent sections of contralateral (A–C) and electroporated (D–F) limbs were immunostained with MF20 and labeled with Hoechst to visualize nuclei in blue (A,D) or hybridized with ANKRD1 (B,E) and JAG2 (C,F) probes. (D) Tomato indicates the electroporated muscle fibers. (A–F) ANKRD1 (E) and JAG2 (F) expression was activated in paralyzed muscles in the presence of YapS112A (D) in differentiated muscle cells compared to paralyzed muscles (A–C). (I) Tomato expression in wholemount MLC-Tomato-YapS112A-electroporated limbs. (G,H,J–L) Transverse sections of contralateral left (G,J) and MLC-Tomato-YapS112A electroporated right (H,K,L) limbs of DMB-treated fetuses were co-immunostained with PAX7 and MF20 antibodies. (L) Tomato expression in the same limb section as (H,K) to visualize electroporated muscle fibers. (M) High magnifications of dorsal muscle areas of DMB and DMB+YapS112A fetuses showing PAX7/TOMATO/HOECHST and MF20/HOECHST. (N) PAX7+ cell number was counted per unit area in muscles displaying Tomato expression in electroporated limbs and in equivalent muscles of contralateral limbs of four embryos. Results are shown as percentage of control. Error bars indicate standard deviations. The p-value was obtained using the Mann-Withney test. Limb sections are dorsal to the top and posterior to the left. u, ulna, r, radius.
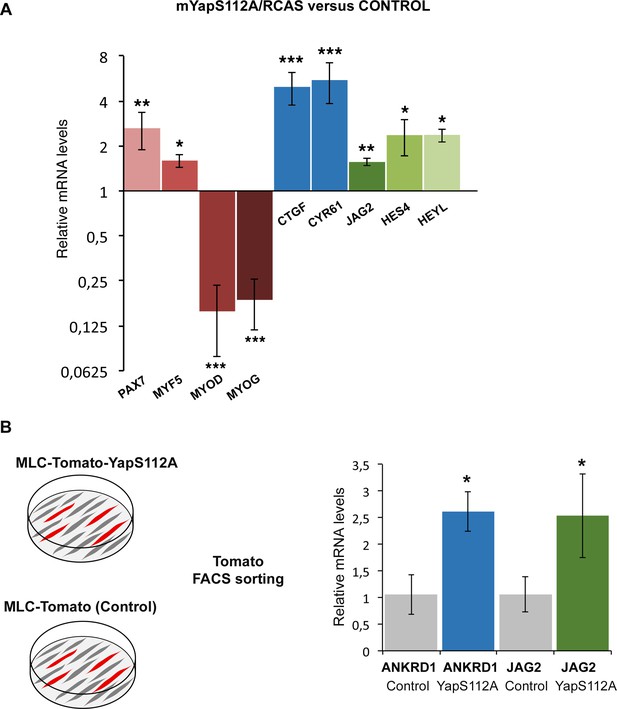
Forced YAP activity increases JAG2 expression in chick fetal myoblasts.
(A) RT-q-PCR analysis of the mRNA levels of muscle genes, YAP target genes and components of the NOTCH pathway in primary cultures of fetal myoblasts transfected with YapS112A/RCAS (N = 6). Graph shows means ± standard errors of the means. For each gene, the mRNA levels of cultured fetal myoblasts transfected with a control vector (Empty/RCAS) was normalized to 1. The relative expression levels of YAP target genes CTGF and CYR61 were significantly increased. The relative expression levels of PAX7 and MYF5 were increased, while those of MYOD and MYOG were downregulated. The expression levels of the NOTCH ligand JAG2 were increased upon forced YAP activation as those of the HES4 and HEYL compared to control cultures. (B) RT-q-PCR analysis of the mRNA levels of ANKRD1 and JAG2 of differentiated muscle cells obtained after Tomato FACS-sorting of primary cultures of fetal myoblasts after transfection of MLC-Tomato-YapS112A. For each gene, the mRNA levels of cultured fetal myoblasts transfected with a MLC-Tomato was normalized to 1. The p-values were calculated using the Mann-Whitney test. Asterisks indicate the p-value, *p<0.05; **p<0.01; ***p<0.001.
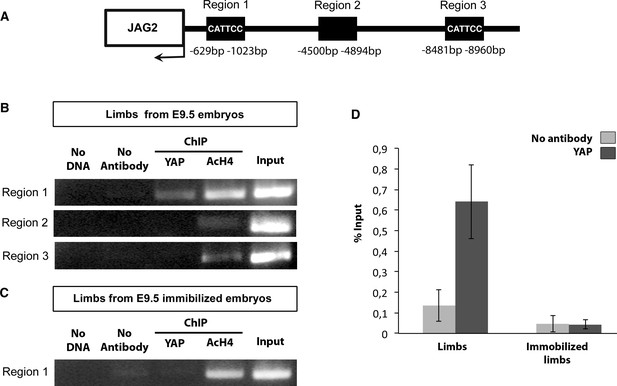
YAP is recruited to a MCAT element-containing promoter region of the chick JAG2 gene in fetal muscles upon muscle contraction.
ChIP assay was performed from eight limbs of E9.5 chick control or immobilized fetuses with antibodies against YAP, AcH4 for positive control, or without antibody as a negative control in two independent biological experiments. ChIP products were analyzed by PCR (B,C) (N = 4) or by RT-q-PCR (N = 2) (D). Primers targeting a 394 pb fragment named region 1 (−629 bp −1023 bp) in the JAG2 promoter region (A) identified a DNA sequence immunoprecipitated by YAP by PCR (B) or by RT-q-PCR (D), while primers targeting regions 2 and 3 did not show any immunoprecipitation by PCR (B). (D) Experiment showing the signal of relative YAP recruitment to JAG2 regulatory region 1 in control and immobilized limbs. Results were represented as percentage of the input. Error bars show standard deviations. The YAP recruitment to JAG2 regulatory region 1 was lost in the absence of muscle contractions assessed by PCR (C) and RT-q-PCR (D) analyses.
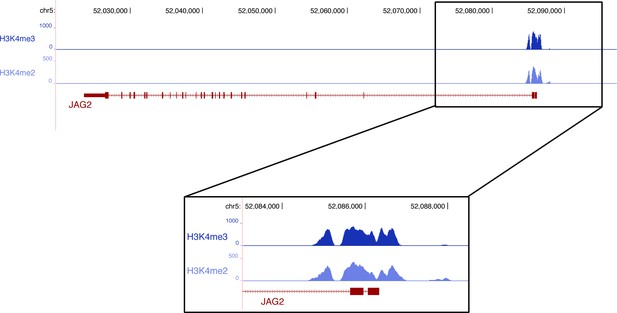
ChiP sequencing data with promoter histone marks on the chick JAG2 gene.
ChiP sequencing was performed from chick limb cell cultures with H3K4me2 and H3Kme3, which are promoter and active promoter marks, respectively.
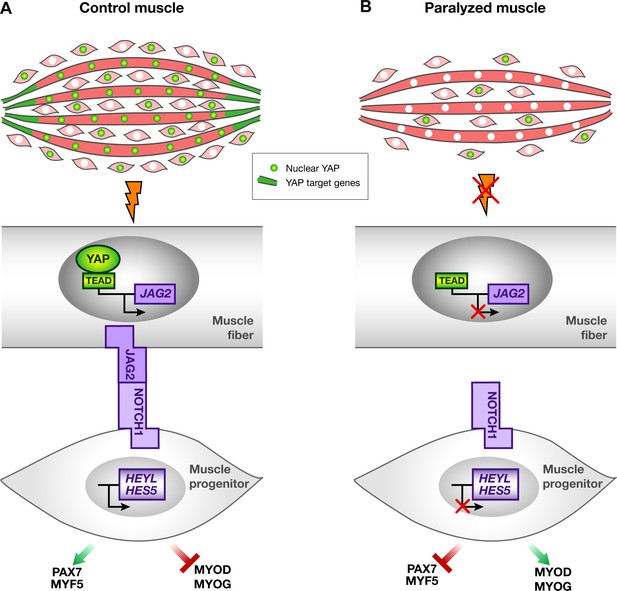
Schematic representation of YAP and NOTCH signaling pathways in normal contracting muscles and paralyzed muscles.
(A) In contracting muscles, nuclear YAP (green myonuclei) and YAP target gene transcripts (green) are present in post-mitotic muscle fibers. YAP positively regulates JAG2 transcription upon muscle contraction. Ligand-dependent NOTCH activation regulates the muscle progenitor pool, by preventing muscle progenitors to differentiate. (B) In paralyzed muscles, nuclear YAP, YAP target genes and JAG2 transcripts are lost in post-mitotic muscle fibers. The absence of the NOTCH ligand JAG2 in fibers, due to the loss of mechanical signals, induces a NOTCH loss-of-function phenotype i.e. a diminution in the number of muscle progenitors and a shift toward differentiation, in a non-cell autonomous manner.
Additional files
-
Supplementary file 1
List of primers used for RT-q-PCR analyses and ChIP assays.
- https://doi.org/10.7554/eLife.15593.018





