Tim29 is a novel subunit of the human TIM22 translocase and is involved in complex assembly and stability
Figures
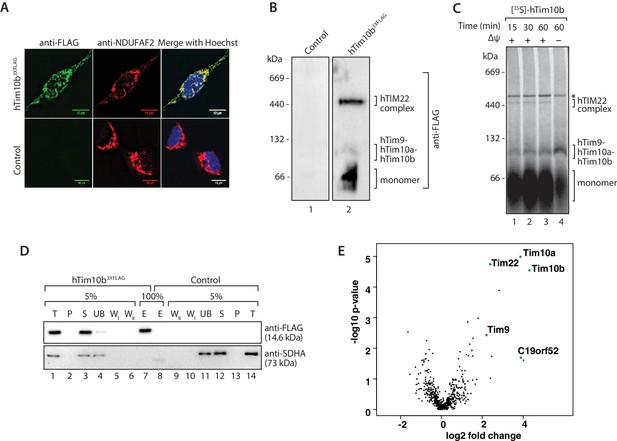
The previously uncharacterised protein C19orf52 immunoprecipitates with hTim10b.
(A) Representative fluorescence images of tetracycline-induced HEK293T cells that contained the Control empty vector (pCDNA5-FRT/TO) or hTim10b3XFLAG. Cells were fixed prior to incubation with anti-FLAG (left panel, green; to stain hTim10b3XFLAG) and anti-NDUFAF2 (middle panel, red; to visualise the mitochondria). Hoechst stain (far right panel, blue) was used to stain the nucleus. Primary antibodies were counterstained with Alexa Fluor 568 and 488 secondary antibodies prior to microscopy. Scale bar: 10 µm. (B) Mitochondria isolated from control cells or cells expressing hTim10b3XFLAG were solubilised in digitonin-containing buffer before being analysed by BN-PAGE and immunoblotting using anti-FLAG antibodies. (C) [35S]-labelled hTim10b precursor was imported into mitochondria isolated from wild type HeLa cells for the indicated times in the presence or absence of a membrane potential (Δψ). Following import the mitochondria were re-isolated, lysed in digitonin-containing buffer and subjected to blue native electrophoresis and autoradiography. Asterisk (*) indicates non-specific band. (D) Mitochondria isolated from control and hTim10b expressing cells were solubilised in digitonin-containing buffer. Mitochondrial lysates were subjected to immunoprecipitation with anti-FLAG resin. Collected fractions were analysed by SDS-PAGE and western blotting using anti-FLAG and anti-SDHA antibodies. T, Total; P, Pellet; S, Supernatant; UB, Unbound; WI and WII, Wash I and II and E, Elution. 5% of the T, P, S, UB, WI and WII fractions and 100% of the E fraction were loaded for SDS-PAGE analysis. (E) Volcano plot showing proteins enriched in hTim10b pull-down versus the empty vector control. All proteins were plotted and each circle represents one protein/gene. The X-axis shows the Log2 fold change of Tim10b interacting partners and Y-axis shows the −log10 of the p-values.
-
Figure 1—source data 1
Data from Figure 1E.
Rows are ordered by t-test difference between empty vector and hTim10b3xFLAG triplicates.
- https://doi.org/10.7554/eLife.17463.004
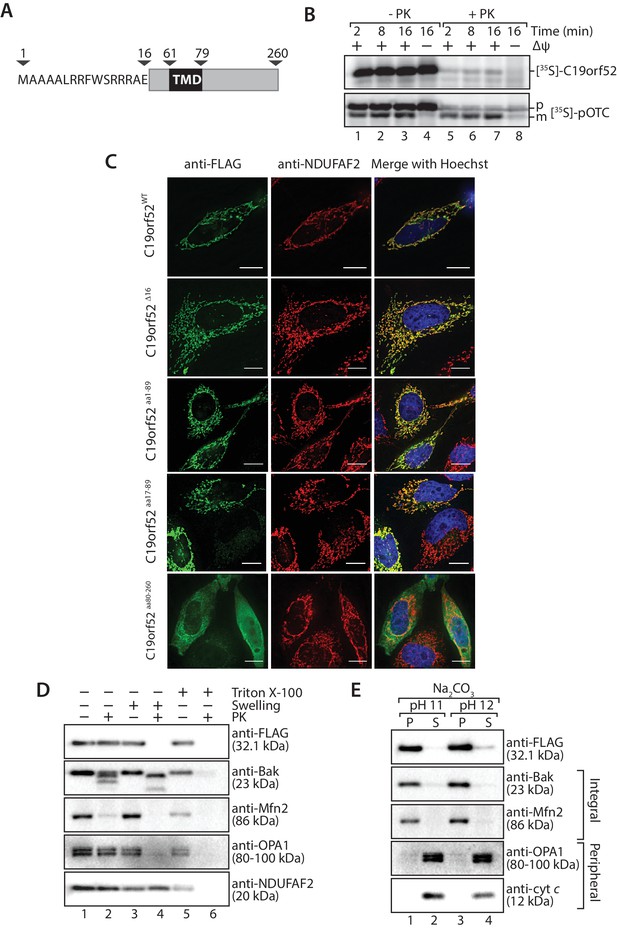
C19orf52 is a mitochondrial inner membrane protein.
(A) Schematic representation of the predicted domain structure for C19orf52 from Homo sapiens. (B) In vitro import of [35S]-labelled C19orf52 and pre-ornithine transcarbamylase, pOTC into mitochondria isolated from HeLa cells for the indicated times in the presence or absence of membrane potential (Δψ). Following import mitochondria were re-isolated and either left untreated (lanes 1–4) or treated with Proteinase K (lanes 5–8). Samples were analysed using SDS-PAGE and autoradiography. p, precursor and m, mature. (C) The indicated C19orf52 variants (C19orf52WT, C19orf52Δ16, C19orf52aa1-89, C19orf52aa17-89 and C19orf52aa80-260) were transiently transfected and expressed as C-terminal 3XFLAG fusions in HeLa cells. Cells were immunostained with anti-FLAG and anti-NDUFAF2 (mitochondria marker) antibodies for visualisation using fluorescence microscopy. Scale bar: 10 µm. (D) Mitochondria were isolated from stable tetracycline-inducible HEK293T cells expressing C19orf523XFLAG. Intact mitochondria (lanes 1 and 2), mitoplasts (generated by hypotonic swelling of the outer membrane, lanes 3 and 4) and solubilised mitochondria (Triton X-100, lanes 5 and 6) were incubated with or without Proteinase K (50 µg/ml) and analysed by SDS-PAGE and western blotting using the indicated antibodies. (E) Mitochondria isolated from C19orf523XFLAG expressing cells were subjected to alkaline extraction using 100 mM Na2CO3 (pH 11 and 12). The membrane (P) and soluble (S) fractions were separated by ultra-centrifugation prior to SDS-PAGE and immunoblotting analysis using the indicated antibodies.

C19orf52 is conserved in metazoa.
Alignment of C19orf52 homologous proteins from the indicated metazoa species using ClustalW2. Dark grey indicates 100% identity, light grey either 80% or 60% identity (conserved in 4 or 3 species, respectively).
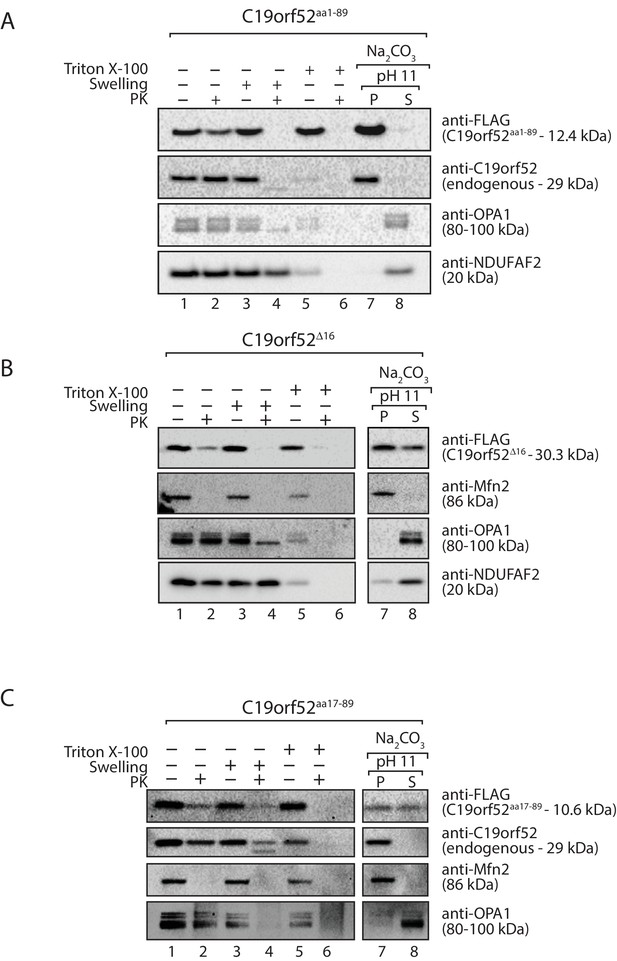
C19orf52 lacking the N-terminal 16 amino acids is not sorted to the inner membrane.
Mitochondria were isolated from cells transiently transfected with DNA encoding (A) C19orf52aa1-89 (B) C19orf52△16 and (C) C19orf52aa17-89 as 3XFLAG fusions. Mitochondria (lanes 1 and 2), mitoplasts (lanes 3 and 4) and solubilised mitochondrial membranes (lanes 5 and 6) were left untreated (lanes 1, 3 and 5) or treated with Proteinase K (lanes 2, 4 and 6). Intact mitochondria were also treated with sodium carbonate to separate mitochondrial proteins into membrane integrated (pellet, P) or peripheral (supernatant, S) proteins. Samples were analysed by SDS-PAGE and western-blotting.
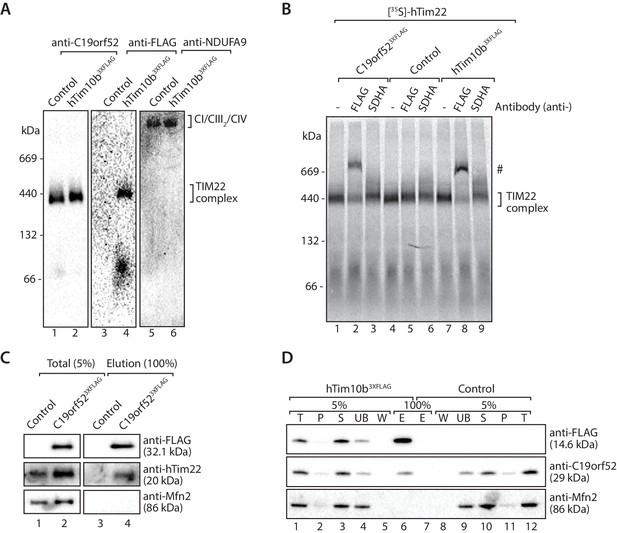
C19orf52 is a subunit of the human TIM22 complex.
(A) Mitochondria isolated from control or hTim10b3XFLAG-expressing cells were solubilised in 1% digitonin-containing buffer and analysed by BN-PAGE and western blotting with the indicated antibodies. (B) [35S]-labelled hTim22 was imported into mitochondria isolated from control, C19orf523XFLAG or hTim10b3XFLAG expressing cells. Following import at 37°C mitochondria were reisolated, solubilised in digitonin-containing buffer and incubated with either anti-FLAG or anti-SDHA antibodies. Samples were separated by BN-PAGE and analysed by autoradiography. # indicates antibody-shifted protein complex. (C) Control and C19orf523XFLAG mitochondria were solubilised in digitonin-containing buffer and subjected to immunoprecipitation using anti-FLAG resin. Total (5%) and Elution (100%) fractions were analysed using SDS-PAGE and immunoblotting. (D) Digitonin-solubilised mitochondrial lysates from control and hTim10b3XFLAG expressing cells were subjected to immunoprecipitation with anti-FLAG resin. Fractions were analysed by SDS-PAGE and western blotting using the indicated antibodies. T, Total; P, Pellet; S, Supernatant; UB, Unbound; W, Wash and E, Elution.
-
Figure 3—source data 1
C19orf52 immunoprecipitates TIM22 complex subunits.
Data from C19orf523XFLAG immunoprecipitations looking at the presence of TIM22 complex subunits. The fold increase (C19orf52/EV) is shown in the last column.
- https://doi.org/10.7554/eLife.17463.009
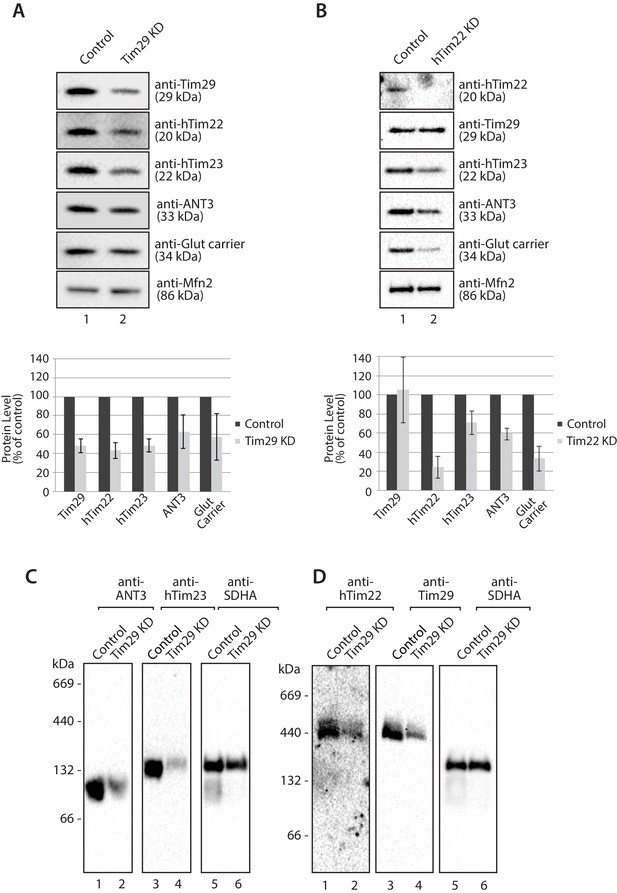
Knockdown of Tim29 reduces the steady state protein levels of TIM22 substrates.
(A) HEK293FS cells were transfected with scrambled (control) or Tim29 siRNA targets for 96 hr. Following knock-down mitochondria were isolated from cells and analysed using SDS-PAGE and western blotting analysis with the indicated antibodies. Protein levels were quantified and normalised against the loading control, Mfn2. The amount of each protein in control cells was set to 100%. Data are expressed as mean ± SD, n = 3. (B) Mitochondria were isolated from control cells (scrambled siRNA) or cells transfected with hTim22 siRNA target for 96 hr. Mitochondrial proteins were subjected to SDS-PAGE and immunoblotting using the indicated antibodies and quantified as described above (C & D) Mitochondria were isolated from control or Tim29 knock down (KD) cells and solubilised in 1% digitonin-containing buffer. Protein complexes were analysed by BN-PAGE and western blotting.
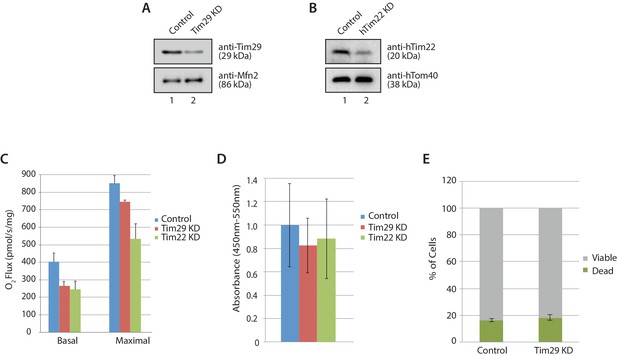
Cellular consequences of Tim29 depletion.
(A) HEK293FS cells were transfected with scrambled (control) or Tim29 siRNA targets for 72 hr. Following knock-down mitochondria were isolated from cells and analysed using SDS-PAGE and western blotting analysis with the indicated antibodies. (B) HEK293FS cells were transfected with scrambled (control) or hTim22 siRNA targets for 72 hr. Following knock-down mitochondria were isolated from cells and analysed using SDS-PAGE and western blotting analysis with the indicated antibodies. (C) Oxygen consumption was measured in HEK293FS cells following knockdown of Tim29 or hTim22 (as described in A and B). Cells transfected with a scrambled siRNA were used as the control. Knockdown of either Tim29 or hTim22 significantly reduced both basal and maximal mitochondrial oxygen consumption rates. Data expressed as mean ± S.D., n = 3. (D) Proliferation of control, Tim29 and hTIm22 KD cells were determined by BrdU incorporation for 24 hr. The level of BrdU incorporation was measured at 450–550 nm (background). Data reported as mean ± SD (n = 3). (E) Cells transfected with scrambled (control) or Tim29 siRNA targets were treated with propidium iodide (PI) 72 hr post transfection and analysed using flow cytometry for% of cell death. Data are shown as mean ± SD (n = 3).
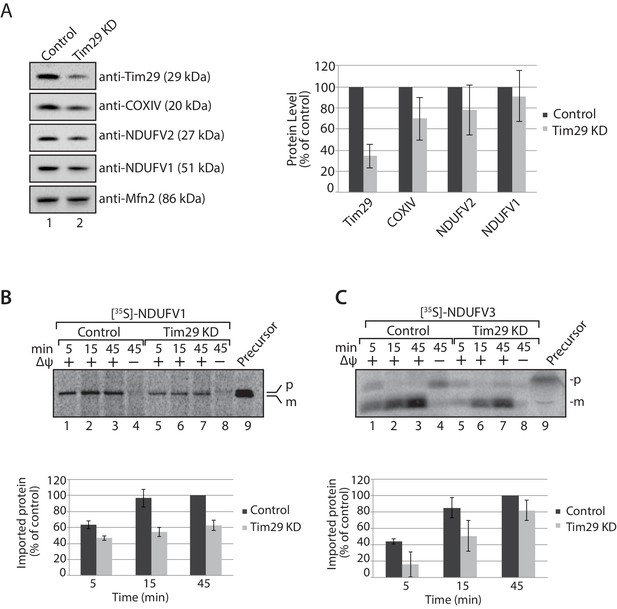
TIM23 complex substrates are reduced in cells depleted of Tim29.
(A) Mitochondria were isolated from control cells (scrambled siRNA) or cells transfected with Tim29 siRNA target for 72 hr. Mitochondrial proteins were subjected to SDS-PAGE and immunoblotting using the indicated antibodies. The levels of the TIM23 complex substrates, COXIV, NDUFV2 and NDUFV1 was quantified relative to protein levels in control mitochondria and normalised against the loading control Mfn2. Data are shown as mean ± SD (n = 3). (B and C) [35S]-labelled NDUFV1 and NDUFV3 were imported into mitochondria isolated from control and Tim29 knockdown cells for the indicated times. Re-isolated mitochondria were treated with protease and separated by SDS-PAGE and analysed by autoradiography. For quantification of imported NDUFV1 and NDUFV3, the longest incubation time (45 min) was set to 100%. Data are shown as mean ± SD (n = 2, NDUFV1; n = 3 NDUFV3). p, precursor and m, mature.
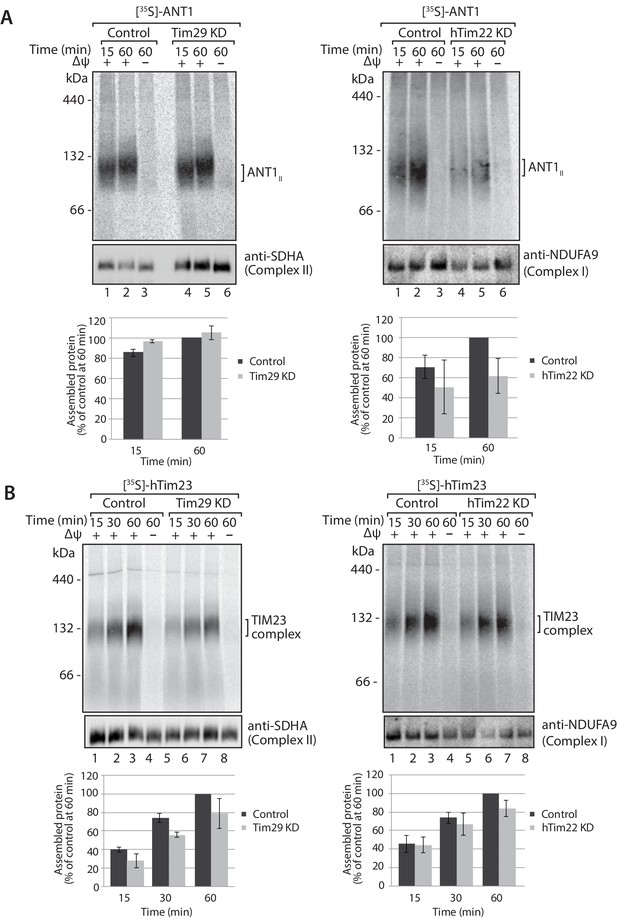
Knockdown of Tim29 influences the assembly of the TIM23 complex, but not ANT1.
(A) [35S]-labelled ANT1 was imported into mitochondria isolated from control or Tim29 (left panel) or hTim22 (right panel) knock-down (KD) cells for the indicated times in the presence or absence of membrane potential (Δψ). Re-isolated mitochondria were treated with 50 µg/ml of Proteinase K prior to solubilisation in digitonin-containing buffer. Mitochondrial lysates were analysed by BN-PAGE and autoradiography. Assembled ANT1 was quantified from three independent experiments. The amount of assembled ANT1 in control mitochondria at 60 min was set to 100%. Data are shown as mean ± SD (n = 3). (B) Import of [35S]-hTim23 was performed and quantified as described above.
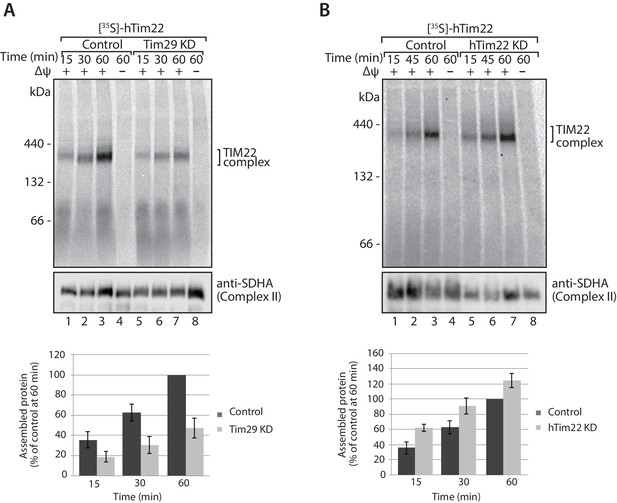
Tim29 is required for the assembly of hTim22 into the mature TIM22 complex.
(A) [35S]-hTim22 was imported into mitochondria isolated from control (scrambled siRNA) or Tim29 knock-down (KD) cells for the indicated times in the presence or absence of membrane potential (Δψ). Re-isolated mitochondria were treated with 50 µg/ml of Proteinase K prior to solubilisation in digitonin-containing buffer. Mitochondrial lysates were analysed by BN-PAGE and autoradiography. Assembled hTim22 was quantified from three independent experiments. The amount of assembled hTim22 in control mitochondria at 60 min was set to 100%. Data are shown as mean ± SD (n = 3). (B) [35S]-hTim22 was imported into mitochondria isolated from control (scrambled siRNA) or hTim22 knock-down cells and treated as described in (A). The amount of assembled hTim22 in control mitochondria at 60 min was set to 100%. Data are represented as mean ± SD (n = 3).
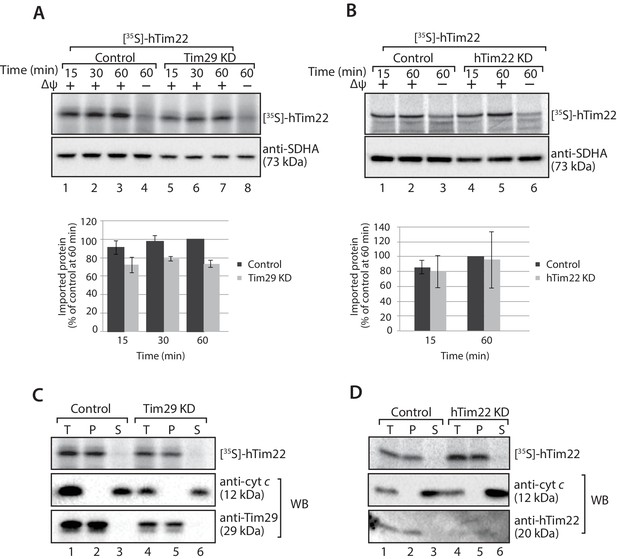
hTim22 is integrated into the inner membrane in the absence of Tim29.
(A and B) [35S]-hTim22 was imported into mitochondria isolated from control (scrambled siRNA), and Tim29 (A) or hTim22 (B) knock-down cells for the indicated times in the presence or absence of membrane potential (Δψ) and analysed by SDS-PAGE. The import efficiency of [35S]-hTim22 into control or Tim29/hTim22 knock-down mitochondria was quantified (mean ± SD, n = 3) and the yield at the longest import time into the control mitochondria was set to 100%. (C and D) [35S]-hTim22 was imported into mitochondria isolated from control (scrambled siRNA), and Tim29 (C) or hTim22 (D) knock-down cells for 45 min. Mitochondria were isolated and treated with sodium carbonate and separated into a membrane integrated (pellet, P) and peripheral membrane protein (supernatant, S). WB, indicates western blot.
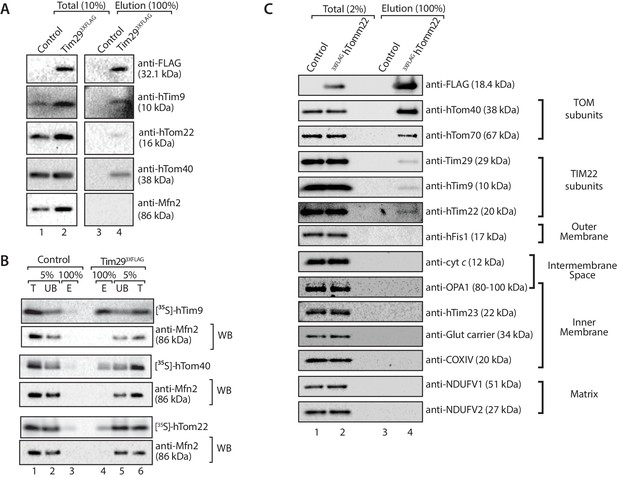
Tim29 couples the TIM22 complex to the TOM complex.
(A) Mitochondria were isolated from control and Tim293XFLAG expressing cells and were solubilised in 0.5% digitonin-containing buffer prior to immunoprecipitation with anti-FLAG resin. Total (10%) and Elution (100%) fractions were collected and analysed using SDS-PAGE and immunoblotting using the indicated antibodies. (B) [35S]-hTim9, [35S]-hTom40 and [35S]-hTom22 were imported into mitochondria isolated from control cells or cells expressing Tim293XFLAG for 60 min. Following import samples were solubilised in 0.5% digitonin-containing buffer prior to immunoprecipitation with anti-FLAG resin. Total (T; 5%), Unbound (UB; 5%) and Elution (E; 100%) fractions were separated on SDS-PAGE followed by autoradiography and western blotting using Mfn2 antibody. (C) Control and 3xFLAGhTom22-harbouring mitochondria were lysed in 0.5% digitonin-containing buffer. Mitochondrial extracts were subjected to immunoprecipitation and were separated on SDS-PAGE prior to immunoblotting analysis. Total, 2% and Elution, 100%.
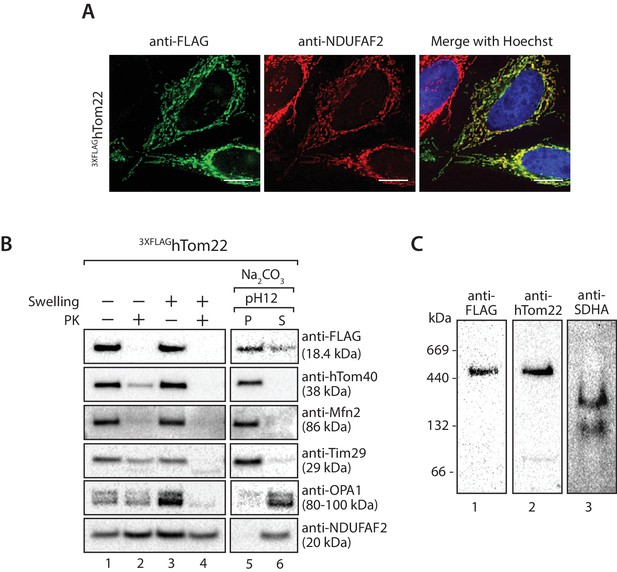
3XFLAGhTom22 localises to mitochondria and assembles within the TOM complex.
(A) HeLa cells were transiently transfected with DNA encoding 3XFLAGhTom22 and analysed by immunofluorescence using anti-FLAG antibodies (green) and anti-NDUFAF2 (mitochondrial marker- red). Cells were visualisation using fluorescence microscopy. Scale bar: 10 µm. (B) Mitochondria were isolated from cells transiently transfected with DNA encoding 3XFLAGhTom22. Mitochondria (lanes 1 and 2) and mitoplasts (lanes 3 and 4) were left untreated (lanes 1 and 3) or treated with Proteinase K (lanes 2 and 4). Intact mitochondria were also treated with sodium carbonate to separate mitochondrial proteins into membrane integrated (pellet, P) or peripheral (supernatant, S) proteins. Samples were analysed by SDS-PAGE and western blotting. (C) Mitochondria isolated from 3XFLAGhTom22 expressing cells were solubilised in digitonin-containing buffer and analysed by BN-PAGE and immunoblotting with the indicated antibodies.
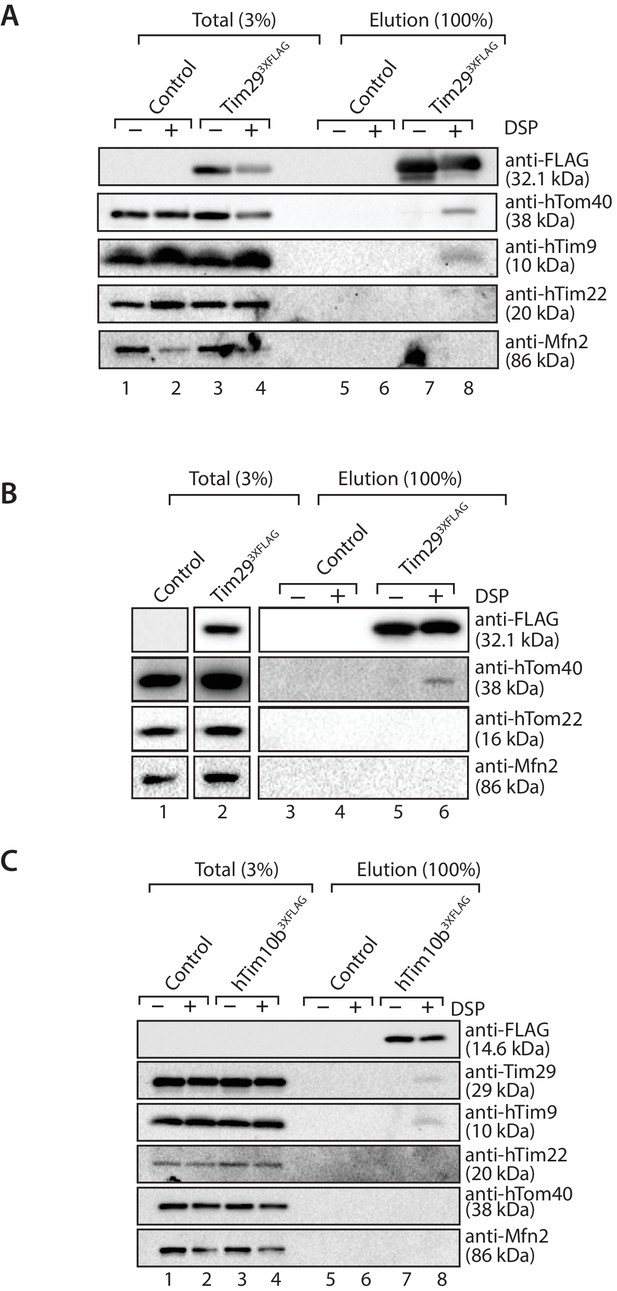
Tim29 interacts with hTom40.
(A and B) Mitochondria isolated from control and Tim293XFLAG expressing cells were incubated with 0.2 mM DSP. Upon quenching of excess cross-linker, mitochondria were solubilised in SDS-containing buffer and subjected to immunoprecipitation using anti-FLAG resin. Cross-linked species were cleaved using the Laemmli buffer. Total (3%) and Elution (100%) fractions were analysed using SDS-PAGE and western blotting with the indicated antibodies. (C) Mitochondria isolated from control and hTim10b3XFLAG expressing cells were treated as described for A and B.





