Ciliary transcription factors and miRNAs precisely regulate Cp110 levels required for ciliary adhesions and ciliogenesis
Figures
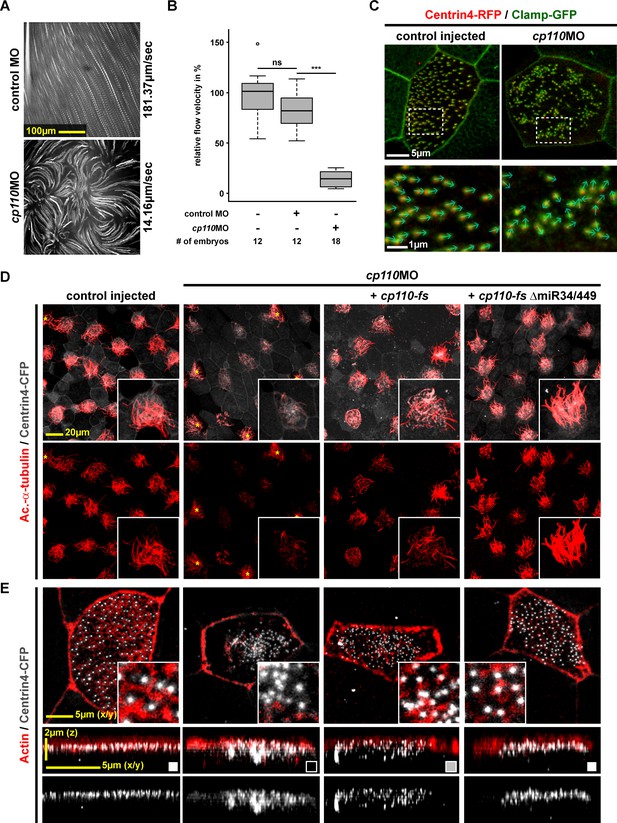
Cp110 is required for basal body function in MCC ciliogenesis.
(A) cp110 knockdown causes impaired extracellular fluid flow. Control (uninjected controls and control MO injected specimens) and cp110MO-injected embryos were analyzed for extracellular fluid flow (10 s projections are shown). (B) Velocities were quantified by particle tracking (Related to Video 1). ***p<0.001; ns, p>0.05 from Wilcoxon two-sample test. (C) Alignment of basal bodies is disrupted in cp110 morphant MCCs. Centrin4-RFP (basal bodies, red), Clamp-GFP (rootlets, green). Arrows in bottom panels show basal body directionality. n embryos/MCCs: control (9/27), cp110MO (10/30). (D) Knockdown of cp110 causes severe defects in MCC ciliogenesis which can be rescued by cp110 DNA co-injection, demonstrated by immunofluorescence for Acetylated-α-tubulin (cilia, Ac.-α-tub., red). Trgeted MCCs were identified by co-injection of centrin4-cfp. Non-targeted MCCs (asterisks) produced normal cilia. (Related to Figure 1—figure supplement 2A). (E) Loss of Cp110 disrupts basal body apical transport and F-actin formation. Basal bodies (Centrin4-CFP, white) and Actin (red) are shown in apical (top row) and lateral (bottom rows) views of individual MCCs. Top views and lateral projections show representative examples (boxes indicate phenotype: white = wt; gray = mild docking defect; black = severe docking defect). (Related to Figure 1—figure supplement 2B,C). See also:
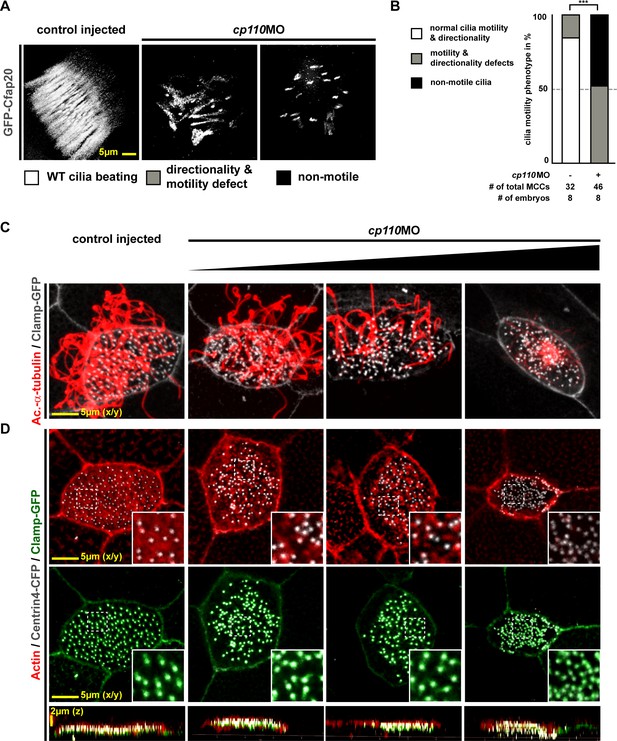
Cp110 is required for basal body function in MCC ciliogenesis.
(A) Cp110-deficient MCC cilia fail to beat directionally. gfp-cfap20 injected embryos were used to visualize ciliary beating (10 s projections are shown). (Related to Video 2–3). (B) Quantification of cilia motility data. ***p<0.001 from χ²-test. (C–D) cp110MO dose-dependent phenotypes of MCC ciliation, basal bodies and apical Actin. cp110MO doses used: 0pmol (control injected; first row), 3pmol (second row), 5pmol (third row), and 7pmol (fourth row). (C) Cilia were visualized by immunofluorescence for Acetylated-α-tubulin (cilia, Ac.-α-tub., red), rootlets were visualized by Clamp-GFP (white). n embryos/MCCs: control (6/19), cp110MO 3pmol (6/22), 5pmol (6/22), 7pmol (6/21). (D) Actin (red), basal bodies (Centrin-CFP, white), rootlets (Clamp-GFP, green). n embryos/MCCs: control (6/22), cp110MO 3pmol (6/17), 5pmol (6/22), 7pmol (6/29).
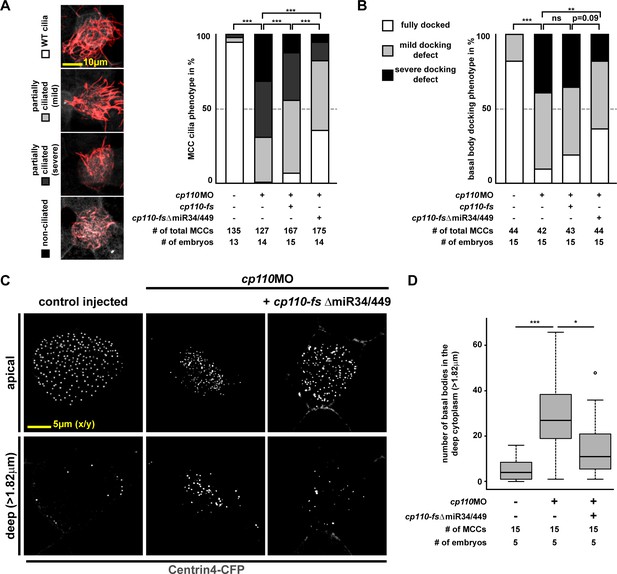
Quantification of basal body and ciliogenesis phenotypes in MCCs.
(A) Quantification of MCC ciliogenesis phenotypes. Color code as indicated in images on the left. ***p<0.001 from χ²-test. Related to Figure 1D. (B–D ) Quantification of basal body phenotypes. (B) Color code as indicated in lateral projection images in Figure 1E. ***p<0.001; **p<0.01; ns, p>0.05 from χ²-test. Related to Figure 1E. (C–D) Representative examples of apically localized and cytoplasmic basal bodies (C) as used for quantification (D) of basal bodies in the deep cytoplasm (>1.82 μm below apical membrane). Samples are derived from one representative experiment included in (B).
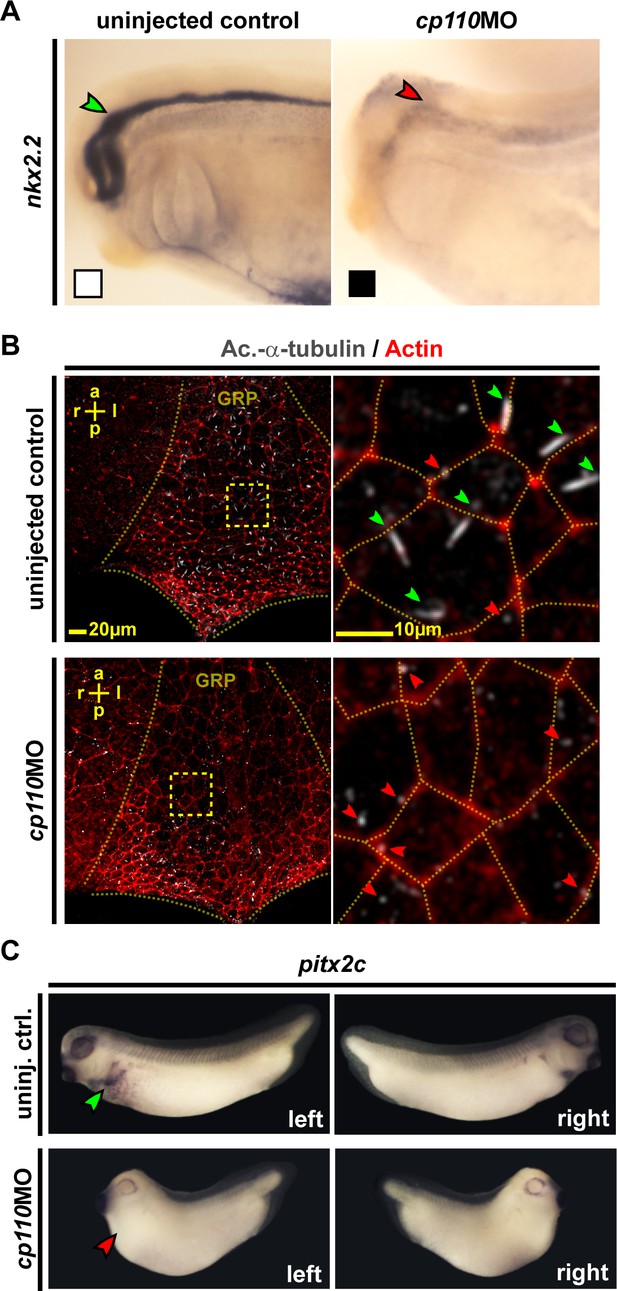
Cp110 is required for primary and motile monocilia.
(A) Cp110 is required for Hedgehog signaling-dependent nkx2.2 expression. Whole-mount in situ hybridization (WMISH) staining for nkx2.2 expression in the neural tube. Normal expression indicated by green arrowhead, reduced expression indicated by red arrowhead. Related to Figure 2—figure supplement 1A–C (white and black boxes indicate normal and reduced expression, respectively in graph Figure 2—figure supplement 1A). (B) Cp110 is required for GRP cilia. Immunofluorescent staining for cilia (Ac.-α-tub., white) and cell borders (Actin, red). Green arrowheads, normal cilia; red arrowheads, defective cilia. Related to Figure 2—figure supplement 1D–F. (C) cp110MO interferes with left side specific pitx2c expression in the lateral plate mesoderm as shown by WMISH. Green arrowhead, normal/left expression; red arrowhead, absent expression. Related to Figure 2—figure supplement 1G. See also:
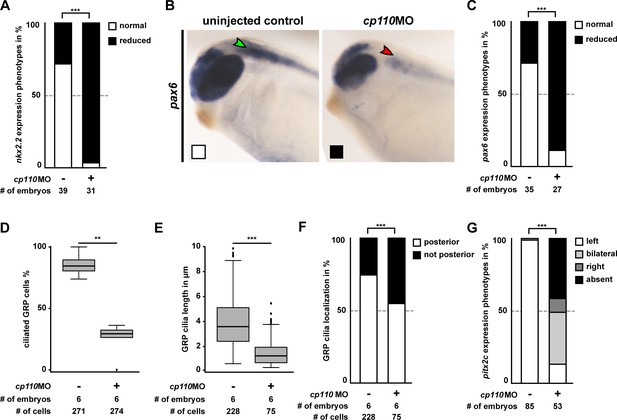
Cp110 is required for primary and motile monocilia.
(A–C) Related to Figure 2A. Control and cp110 morphant embryos were stained by WMISH and cleared embryos were analyzed for nkx2.2 and pax6 expression in the neural tube. (A) Quantification of nkx2.2 expression. (B) WMISH for pax6 expression. Normal expression indicated by green arrowhead, reduced expression indicated by red arrowhead. White and black boxes indicate normal and reduced expression, respectively in graph in C. (C) Quantification of pax6 expression. In (A) and (C), ***p<0.001 from χ²-test. (D–F) Related to Figure 2B. Quantification of GRP ciliation rate (D), GRP cilia length (E), and GRP cilia polarization (F) in central GRP regions from one representative experiment. In (D) and (E), ***p<0.001; **p<0.01; from Wilcoxon two-sample test. In (F), ***p<0.001 from χ²-test. (G) Related to Figure 2 C. Quantification of pitx2c expression. In G, ***p<0.001 from χ²-test.
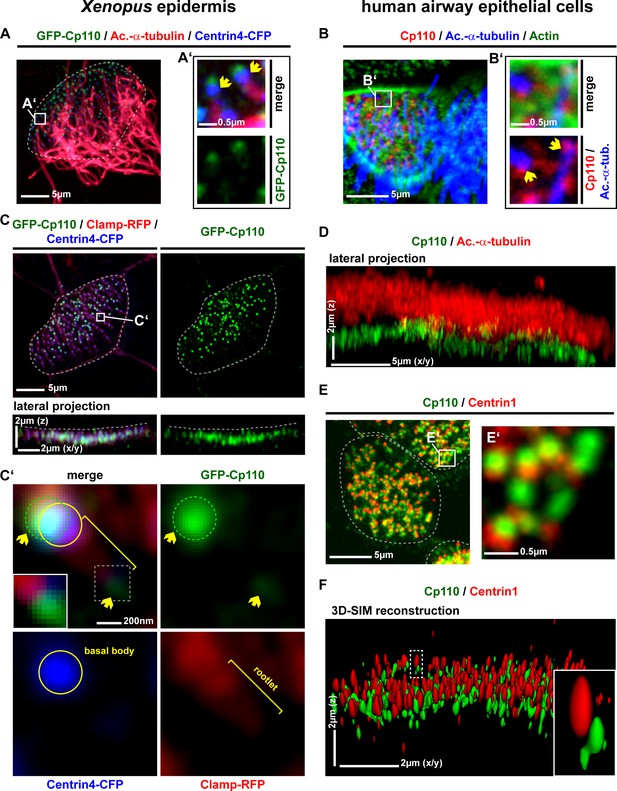
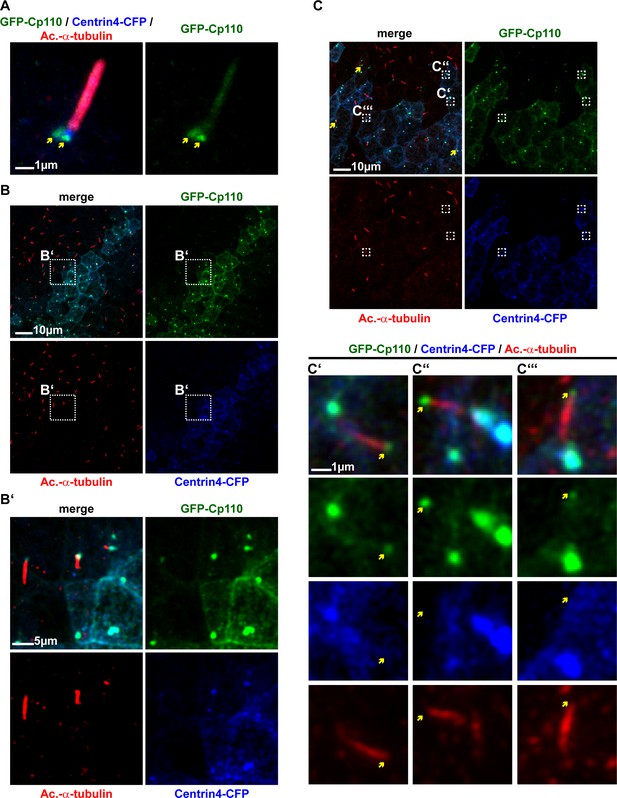
Cp110 localizes to cilia-forming basal bodies in MCCs.
(A–D) Cp110 localizes to cilia-forming basal bodies in Xenopus epidermal (A, C) and human airway epithelial cell (HAEC) (B, D) MCCs. (A) gfp-cp110 (green) was expressed at levels permitting ciliogenesis, together with centrin4-cfp (basal bodies, blue). Immunofluorescent staining (Ac.-α-tub., red) was used to visualize cilia. (B) Immunofluorescent staining for endogenous Cp110 (red), cilia (Ac.-α-tub.; blue) and Actin (green) in MCCs (n donors = 1, n MCCs = 4). Yellow arrows in A’ and B’ indicate the base of cilia. (C) Apical view (top) of individual MCC co-injected with gfp-cp110 (green), centrin4-cfp (blue) and clamp-rfp (red) to visualize Cp110, basal bodies and rootlets, respectively. Localization of basal bodies to the apical membrane is shown in lateral projection (bottom). n embryos/MCCs: (4/18). (C') High-magnification analysis of GFP-Cp110 (green, indicated by yellow arrows and green circle) binding to an individual basal body from the MCC shown in (C) (basal body and rootlet are indicated). Inset shows rootlet domain (dashed box) with increased brightness. (D) Lateral projection of MCC stained for endogenous Cp110 (green) and cilia (Ac.-α-tub.; red). n donors = 1, n MCCs = 12 (same samples as in Figure 3—figure supplement 1C) (E–F) Endogenous Cp110 (green) and Centrin 1 (red) staining shows Cp110 adjacent to MCC basal bodies by confocal microscopy (E) and 3D-SIM imaging (F). n donors = 1, n MCCs = 3 each for confocal and 3D-SIM.
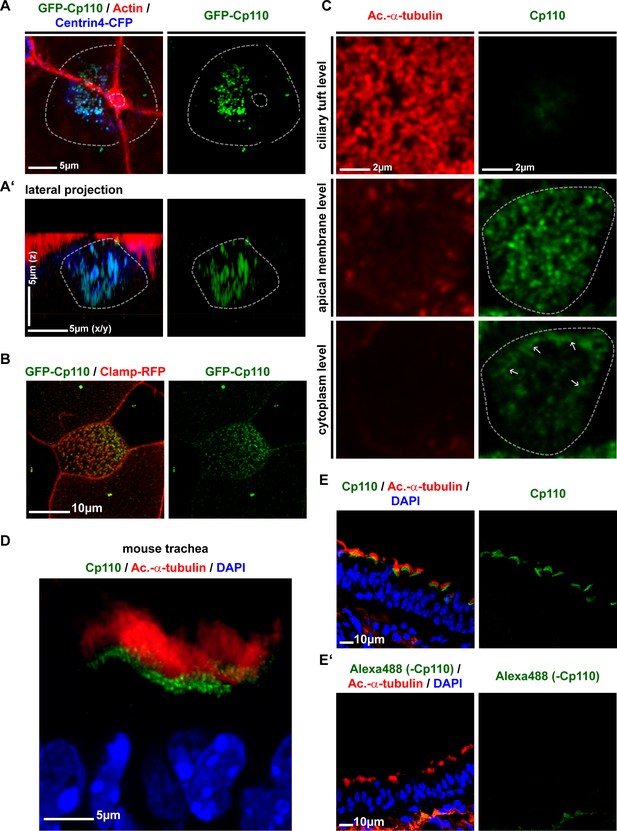
Cp110 localizes to cilia-forming basal bodies in MCCs.
(A–B) GFP-Cp110 localization to basal bodies in Xenopus MCCs. (A) GFP-Cp110 (green) localizes to basal bodies (Centrin4-CFP, blue) prior to apical docking, during the stages of apical basal body transport. Actin staining shown in red. n = 2 embryos, 18 MCCs. (B) GFP-Cp110 (green) shows asymmetric localization to basal bodies/rootlets (Clamp-RFP, red) along the anterior-posterior axis. n = 3 embryos, 30 MCCs. (C) Immunofluorescent staining for Cp110 (green) and cilia (Acetylated-α-tubulin, red) shows Cp110 localization at the level of basal bodies and at the lateral membrane (white arrows) in human HAEC MCCs. Three levels along apical-basal axis are shown (top, apical ciliary tuft level; middle, apical MCC membrane level; bottom, cytoplasmic level). n = 1 donor, 12 MCCs. (D–E) Mouse trachea staining for Cp110 (green), cilia (Acetylated-α-tubulin, red) and nuclei (DAPI, blue). (n = 4). (D) Magnified view of MCCs. (E) Greater area view of mouse trachea with multiple MCCs. (E’) Negative control immunofluorescent staining as described in (E), but without the use of primary anti-Cp110 antibody. (n = 1).
Cp110 localizes to cilia-forming basal bodies and ciliary tips of monociliated GRP cells.
(A–D) GFP-Cp110 localizes to cilia-forming basal bodies in GRP cells injected with gfp-cp110 (green) and centrin4-cfp (basal body/daughter centriole, blue) and immunostained for cilia (Acetylated-α-tubulin, red). (A) Single GRP cilium of normal length (approximately 4 µm) with two GFP-Cp110 foci (yellow arrowheads; mother centriole/basal body and daughter centriole) overlapping with Centrin4-CFP at the base of the cilium. (n = 3). (B), Differential effects of gfp-cp110 expression in GRP cells. Basal bodies (blue) and cilia (red) in GRP cells expressing different amounts of GFP-Cp110 (green). (B’), Magnification of area depicted in (B) showing cilia of different length with different amounts of GFP signal at their base. (C) In some GRPs, a subset of cilia displayed GFP-Cp110 localization to the ciliary tip (boxes and yellow arrows). Three individual cases are shown in (C’–C’’’), where yellow arrows indicate ciliary tip. (n = 9 for B and C combined).
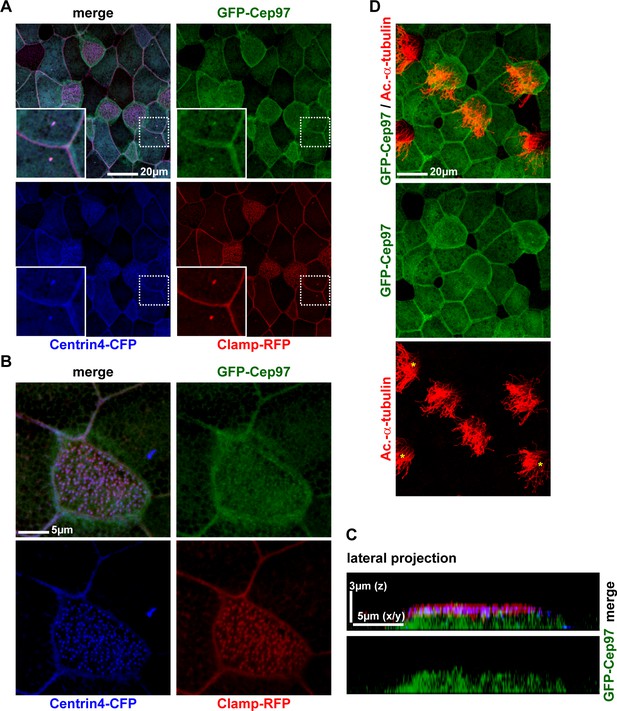
Cep97 does not localize to cilia-forming basal bodies.
(A–C) GFP-Cep97 localizes centrioles of epidermal cells in Xenopus, but does not localize to basal bodies in MCCs. (A) GFP-Cep97 (green) localizes to centrioles (Centrin4-CFP, blue; Clamp-RFP, red) of epidermal cells (inset). n = 3 embryos. (B) GFP-Cep97 (green) does not localize to basal bodies/rootlets in MCCs (Centrin4-CFP, blue; Clamp-RFP, red). n embryos/MCCs: 3/9. (C) Lateral projection of MCC shown in (B) shows primarily cytoplasmic localization of GFP-Cep97. (D) Overexpression of gfp-cep97 does not inhibit cilia in Xenopus MCCs. n embryos/MCCs: 3/15.
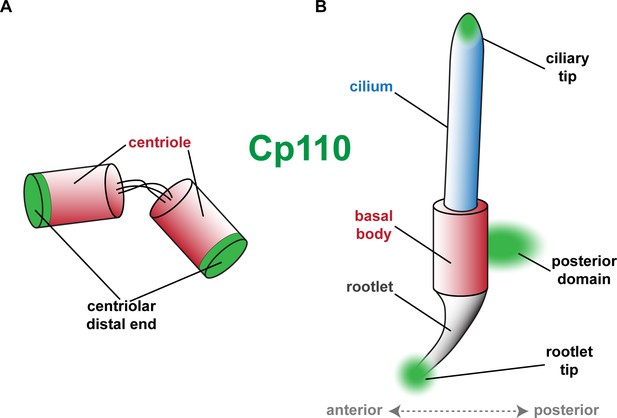
Schematic depiction of Cp110 localization sites at centrioles, basal bodies and cilia.
(A) Cp110 caps the distal ends of centrioles. (B) Cp110 localizes adjacent to the basal body at a posterior domain as well as to the tip of the rootlet. Additionally, Cp110 can localize to ciliary tips.
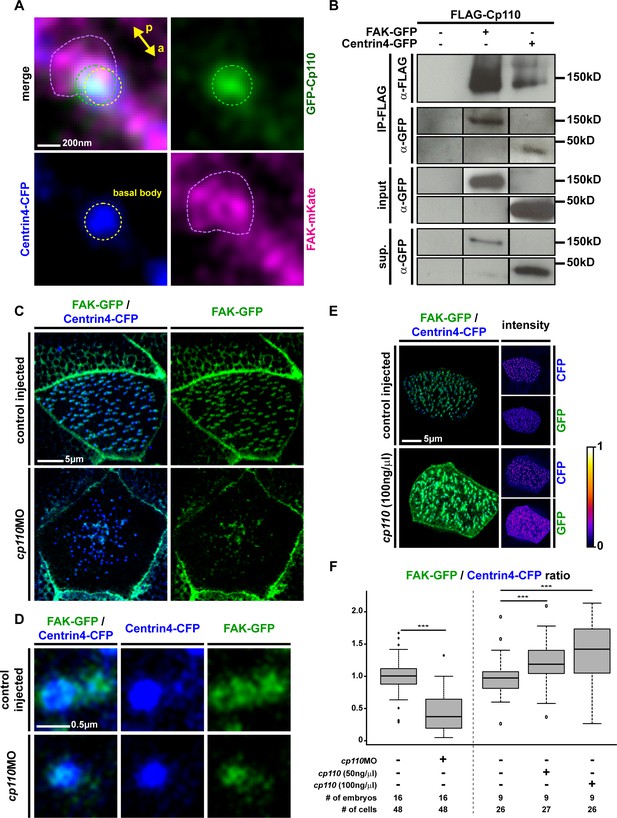
Cp110 is required for ciliary adhesion complex formation in MCCs.
(A) Expression of gfp-cp110 (green) at concentrations permitting ciliogenesis, FAK-mKate (magenta), and centrin4-cfp (blue) revealed polarized posterior localization of GFP-Cp110 and FAK-mKate adjacent to the basal body. Note that FAK-mKate overlaps with GFP-Cp110, but extends past GFP-Cp110 in the posterior direction. n embryos/MCCs (7/28). Related to Figure 4—figure supplement 1A. (B) Western blot analysis of co-immunoprecipitation (co-IP) using Flag-Cp110. FLAG-Cp110 (~140kD) detected by anti-FLAG antibody (α-FLAG). FAK-GFP (~150kD) and Centrin4-GFP (~45kD) detected by anti-GFP antibody (α-GFP). Co-IP, IP-FLAG; input samples, input; supernatant samples, sup. (n = 2). Related to Figure 4—figure supplement 2E. (C–D) Cp110 is required for FAK binding to MCC basal bodies. (C) Mix of FAK-gfp (green) and centrin4-cfp (blue) mRNAs was injected (± cp110MO). Quantification shown in (F). n embryos/MCCs: control (16/48), cp110MO (16/48). (D) Magnification of individual basal body from C. (E) Overexpression of Cp110 caused increased localization of FAK-GFP to basal bodies (Centrin4-CFP, blue). Heatmaps of CFP and GFP intensity levels shown next to merged immunofluorescent images. Color code shown right. Quantification shown in (F). n embryos/MCCs: control (9/26), 50 ng/μl (9/27), 100 ng/μl (9/26). (F) Quantification of FAK-GFP to Centrin4-CFP ratios in controls, cp110 morphants and after overexpression of cp110 (at 50 ng/μl and 100 ng/μl concentrations). ***p<0.001 from Wilcoxon two-sample test.
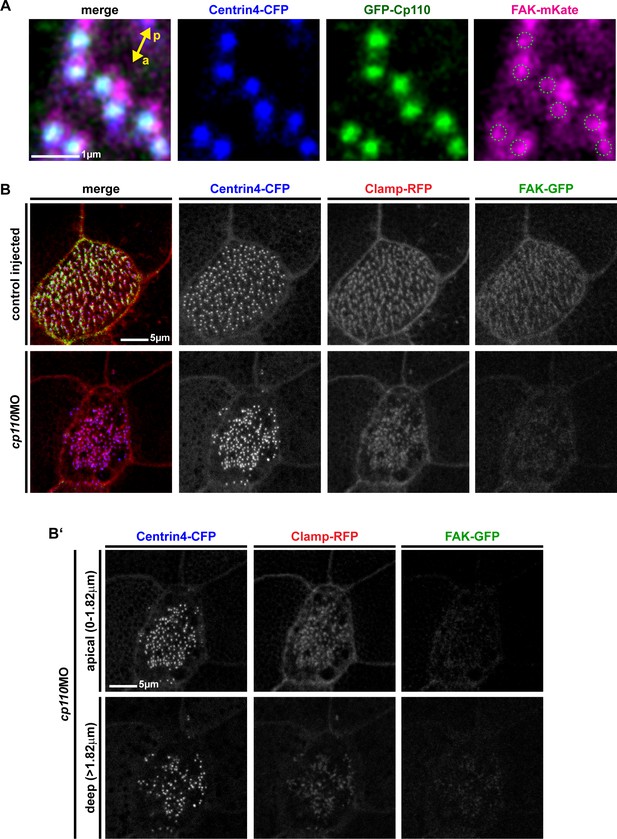
Cp110 is required for ciliary adhesion complex formation in MCCs.
(A) Greater area view of gfp-cp110 (green), FAK-mKate (magenta) and centrin4-cfp (blue) overexpression in MCCs. Green circles in magenta channel indicate position of Cp110. Related to Figure 4A. (B )FAK-GFP (green) levels were greatly reduced in cp110 morphants, while Centrin4-CFP (blue) and Clamp-RFP (red) levels remained largely unchanged. n embryos/MCCs: control (6/18), cp110MO (9/27). (B’) Apical (0–1.82 μm) and deep (>1.82 μm) localized basal bodies are shown from MCC presented in (B).
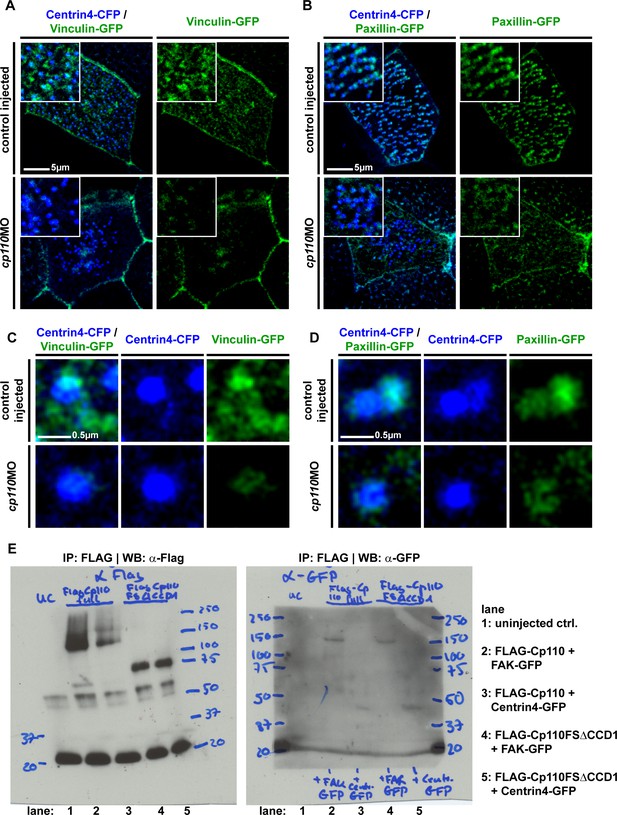
Cp110 is required for ciliary adhesion complex formation in MCCs.
(A-D) Cp110 is required for Vinculin (n embryos/MCCs: control (10/30), cp110MO (10/30)) and Paxillin (n embryos/MCCs: control (5/15), cp110MO (5/15)) localization to MCC basal bodies. Control and cp110 morphant embryos were injected with vinculin-gfp (A) and (C), green) or paxillin-gfp (B) and (D), green) together with centrin4-cfp (blue). Related to Figure 4C–D. (E) Full membranes of co-IP experiment shown in Figure 4B. In addition to FLAG-Cp110 (full length Cp110), a FLAG-Cp110-FSΔCCD1 was overexpressed, which misses the first coiled-coil domain, as well as the truncation of the cp110-fs clone (please compare to Figure 5D).
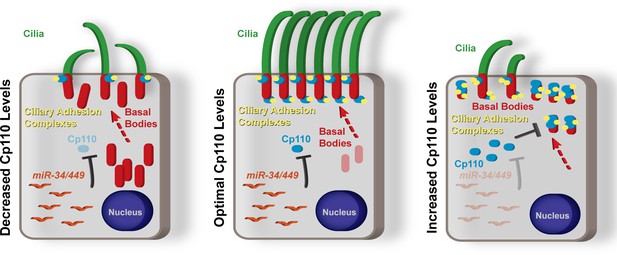
Schematic representation of summary model of the roles of Cp110 in MCC ciliation.
Cp110 levels in MCCs need to be precisely regulated for successful ciliogenesis and normal cilia function. Please see text for detailed description.
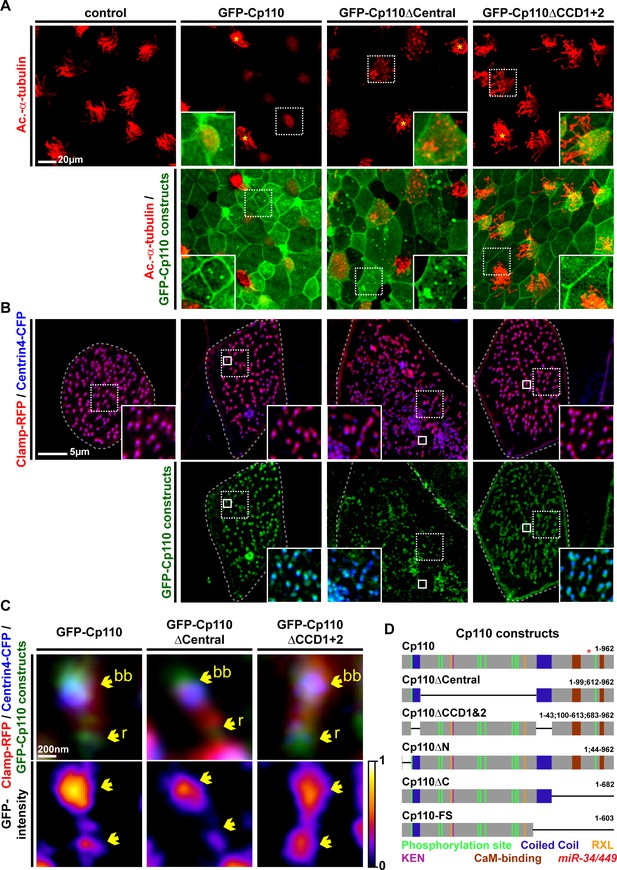
Cp110 coiled-coil domains are required for cilia inhibition and centriolar functions.
(A) Cp110 coiled-coil domains are required for MCC cilia inhibition and formation of supernumerary centrioles. Controls and embryos injected with full-length gfp-cp110, gfp-cp110ΔCentral or gfp-cp110ΔCCD1+2 (all green) were analyzed for ciliation by immunofluorescent staining (Ac.-α-tub., red). Upper panels: red fluorescence channel; merge channel insets show magnifications outlined by dashed boxes. Lower panels: green/red merge; insets show magnifications of non-MCCs outlined by dashed boxes. Related to Figure 5—figure supplement 1A–B. (B) Cp110 coiled-coil domains are required for basal body aggregation. Embryos injected with centrin4-cfp (basal bodies, blue), clamp-rfp (rootlets, red), and either full-length gfp-cp110 or gfp-cp110ΔCentral or gfp-cp110ΔCCD1+2 (green). Upper panels: red/blue merge; insets show magnifications of basal bodies outlined by dashed boxes. Lower panels: green/blue merge; insets show magnifications of basal bodies outlined by dashed boxes. n embryos/MCCs: control (7/21), gfp-cp110 (7/21), gfp-cp110ΔCentral (7/21), gfp-cp110ΔCCD1+2 (7/21). Related to Figure 5—figure supplement 1D. (C) GFP-Cp110 constructs show different localization patterns at basal bodies (bb) and rootlets (r). Upper panels: Individual basal bodies from MCCs shown in (B) (solid boxes). Lower panels: heat maps of GFP-Cp110 intensity. Color code shown right. Related to Figure 5—figure supplement 1D. (D) Cp110 constructs generated in this study. Different colors indicate predicted functional domains. Green, CDK phosphorylation sites; blue, coiled-coil domains; yellow, Cyclin binding domain (RXL); pink, KEN domain (proteasomal degradation); brown, CaM-binding domains; red asterisk indicates the position of miR-34/449 target site in the cp110 mRNA. See also:
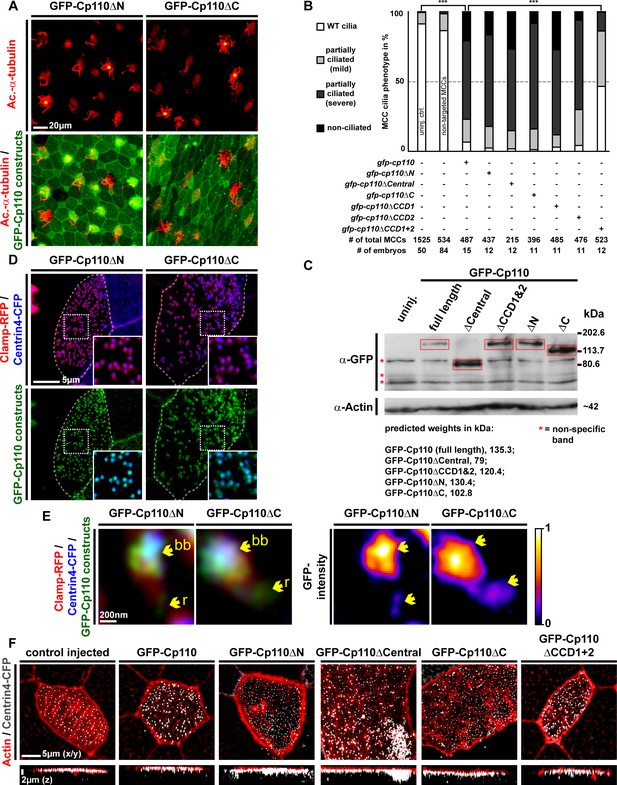
Cp110 coiled-coil domains are required for cilia inhibition and centriolar functions.
(A) Related to Figure 5A. Embryos injected with gfp-cp110ΔN or gfp-cp110ΔC (all green) were analyzed for ciliation by immunofluorescent staining against Acetylated-α-tubulin (cilia, Ac.-α-tub., red). Upper panels: red fluorescence channel only. Lower panels: green/red merge channels. (B) Related to (A) and Figure 5A. Quantification of MCC cilia phenotype in controls and after overexpression of full-length gfp-cp110 and deletion constructs. ***p<0.001 from χ²-test. (C) Immunoblot of GFP-Cp110 (α-GFP antibody) constructs to monitor expression levels. Please note that GFP-Cp110ΔCCD1+2 levels are not reduced as compared to cilia inhibiting constructs. (D) Related to Figure 5B. Embryos were injected with centrin4-cfp (basal bodies, blue), clamp-rfp (rootlets, red), and either gfp-cp110ΔN or gfp-cp110ΔC (all green). Upper panels: red/blue merge channels; insets show magnifications of basal bodies outlined by dashed boxes. Lower panels: green/blue merge channels; insets show magnifications of basal bodies outlined by dashed boxes. n embryos/MCCs: gfp-cp110ΔN (3/9), gfp-cp110ΔC (3/9). (E) Left panels: Individual basal bodies from MCCs shown in (D) as red/green/blue merge channels. Right panels: heat maps showing GFP-Cp110 intensity levels. Basal body (bb), rootlet (r). Color code shown on the right. (F) gfp-cp110 overexpression induces apical Actin defects in MCCs. Embryos were injected with centrin4-cfp (basal bodies, white) and gfp-cp110 constructs (not shown) and stained for Actin (red). Upper panels: apical view on MCCs. Lower panels: lateral projections show localization of basal bodies along the apical-basal axis on MCCs. n embryos/MCCs: control (9/27), gfp-cp110 (7/12), gfp-cp110ΔCentral (6/18), gfp-cp110ΔCCD1+2 (6/18), gfp-cp110ΔN (3/9), gfp-cp110ΔC (3/9).
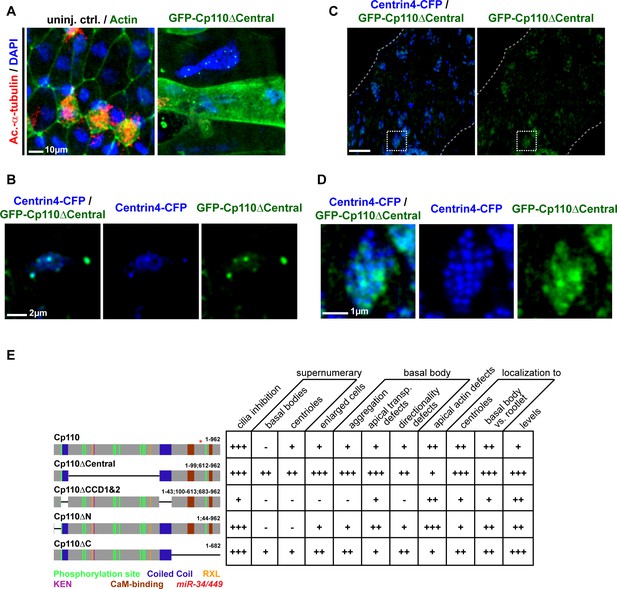
Cp110 central domain deletion enhances centriolar and basal body phenotypes.
(A–E) Related to Figure 5 and Figure 5—figure supplement 1. (A–B) gfp-cp110ΔCentral overexpression induces supernumerary centrioles, polynucleated cells and severe cytokinesis defects. (A) Controls and embryos injected with gfp-cp110ΔCentral (green) were analyzed for ciliation by immunofluorescent staining against Acetylated-α-tubulin (cilia, Ac.-α-tub., red) and nuclei (DAPI, blue). In uninjected control embryos, cell borders were visualized by Actin staining (green, left panel only). n embryos: control, 6; cp110MO, 5. (B) Embryos were injected with centrin4-cfp (centrioles, blue) and gfp-cp110ΔCentral (green). A central region of a non-MCC epidermal cell is shown. All GFP-Cp110ΔCentral foci overlap with Centrin4-CFP foci. (C–D) gfp-cp110ΔCentral overexpression induces increased numbers of basal bodies in MCCs, which frequently fail to separate. (C) Embryos were injected with centrin4-cfp (basal bodies, blue) and gfp-cp110ΔCentral (green), which caused strongly enlarged MCCs and aggregated basal bodies. (D) Magnification of basal body cluster from MCC shown in C. (E) Cp110 constructs generated in this study and their effect on epidermal cells. +, phenotype present; ++, strong phenotype; +++, very strong phenotype; -, phenotype not present.
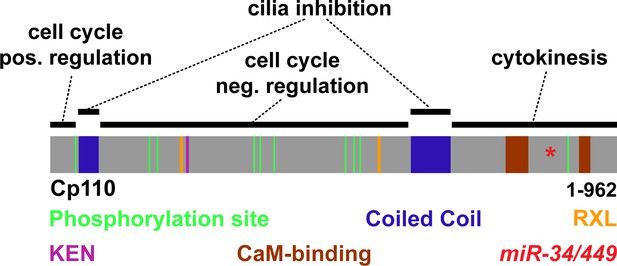
Schematic representation of Cp110 domains and their proposed function.
Cp110 domains are depicted as described in Figure 5D. Proposed functions are indicated.
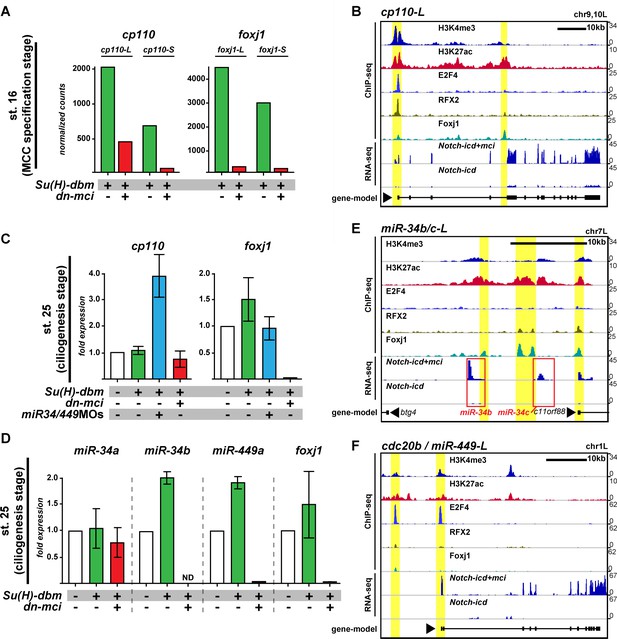
Cp110 levels in MCCs are controlled by ciliary transcription factors and miR-34/449 microRNAs.
(A) cp110 expression in MCCs is regulated through the MCC signaling/transcriptional cascade. Embryos were injected with Su(H)-dbm to stimulate MCC induction (green) or with Su(H)-dbm and dominant-negative multicilin (dn-mci) to prevent MCC induction (red). RNA-sequencing (RNA-seq) was performed at MCC specification stage (st. 16). Normalized counts are shown as bar graphs. n = 2. Related to Figure 6—figure supplement 1A. (B) cp110 expression is activated by ciliary transcription factors. Chromatin immunoprecipitation and DNA-sequencing (ChIP-seq; upper five lanes) and RNA-seq (bottom two lanes) at stage 16. Embryos were injected with Notch-icd to inhibit MCC induction or together with multicilin (mci) to induce MCCs. ChIP-seq using antibodies to mark active chromatin (Histone H3 lysine tri-methylation, H3K4me3; Histone H3 lysine acetylation, H3K27ac), E2F4 binding (E2F4), RFX2 binding (RFX2), and Foxj1 binding (Foxj1) are shown. A gene model is shown in bottom lane. ChIP-seq peaks are indicated by a yellow background. (C) cp110 levels at ciliogenesis stage (st. 25) are controlled by miR-34/449 miRNAs. For quantitative RT-PCR analysis (qPCR), manipulations were performed as described in (A) (green and red bars). Additionally, miR-34/449s were knocked down (miR-34/449MO, blue bar). The uninjected control was set to 1. n = 2. (D) miR-34/449 family members are regulated through the conserved MCC signaling/transcriptional cascade. qPCR analysis for miR-34/449 expression was performed as described (C). ND, not detected. n = 2. (E–F) Expression of miRNAs miR-34b/c and miR-449a-c is activated by ciliary transcription factors. ChIP-seq and RNA-seq was performed as described in (B). miRNA location in (E) is indicated by red box. miR-449a-c are expressed from cdc20b intron 2. Related to Figure 6—figure supplement 1B. The foxj1 expression analysis confirmed successful manipulation in (A, C) and (D). Error bars represent s.e.m. in (C) and (D).
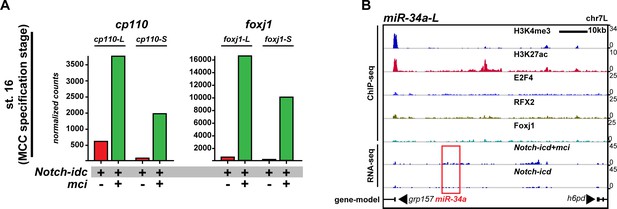
Cp110 levels in MCCs are controlled by ciliary transcription factors and miR-34/449 microRNAs.
(A) Related to Figure 6A. cp110 expression in MCCs is regulated through the conserved MCC signaling/transcriptional cascade. Embryos were injected with Notch-icd to inhibit MCC induction (red) or with Notch-icd together with multicilin (mci) to stimulate MCC induction (green). RNA-sequencing (RNA-seq) was performed on extracts from mucociliary organoids at MCC specification stage (st. 16). Normalized counts are shown as bar graphs. The foxj1 expression analysis confirmed successful manipulation. (B) Related to Figure 6B,E–F. Expression of miRNA miR-34a is not activated by ciliary transcription factors. ChIP-seq and RNA-seq was performed as described in Figure 6B. miRNA location is indicated by red box.
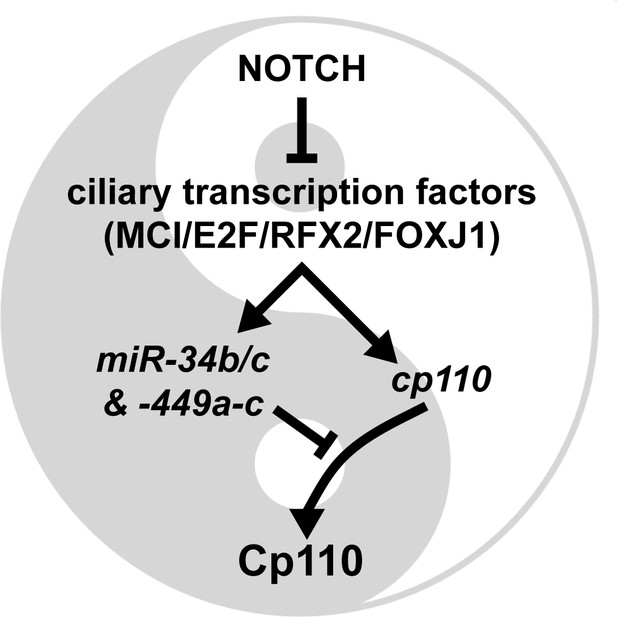
Model of the transcriptional/post-transcriptional regulatory module required to achieve optimal Cp110 levels in MCC ciliogenesis.
A schematic model of ciliary transcription factors and miRNAs is shown. Activation is shown as arrow. Inhibition is shown as T-shaped arrow.
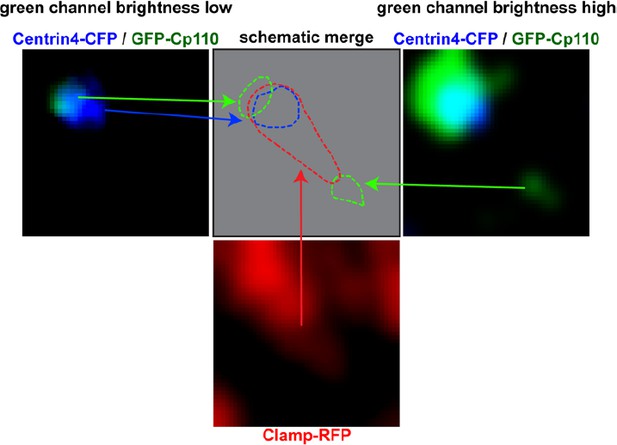
Left panel shows Centrin-CFP (blue) and GFP-Cp110 (green) in images where green channel brightness is low.
In this image Cp110 localizes adjacent to the basal body. Right panel shows Centrin-CFP (blue) and GFP-Cp110 (green) in images where green channel brightness is high. In this image we can visualize the low-level localization of GFP-Cp110 to the rootlet tip, which was not visible in the left panel. Bottom panel shows Clamp-RFP staining of the ciliary rootlet (red). Top row, middle panel shows schematic localization of Cp110 relative to the basal body and the rootlet. Green = Cp110, blue = Centrin4, red = Clamp.

Alignment of the cp110 region, where the missing Adenine was identified in the FS-clone.
BC167469, Xenopus tropicalis genome 9.0 sequence and the current Xenopus tropicalis cp110 reference sequence (XM_0129708) are shown.
Videos
Cp110 is required for extracellular fluid flow in the Xenopus mucociliary epidermis.
Extracellular fluid flow over the Xenopus embryonic epidermis was analyzed at stage 32 by time-lapse imaging of fluorescent beads. Knockdown of cp110 caused severely reduced fluid flow velocity (cp110MO; 14.16 µm/s) and loss of directionality, as compared to control MO-injected (CoMO; 181.37 µm/s) and uninjected (uninj. ctrl.; 228.72 µm/s) specimens. Movie plays at 1x speed. Related to Figure 1A.
Cp110 is required for metachronal synchronous ciliary beating in MCCs.
Embryos were injected with cfap20-gfp to visualize ciliary axonemes of epidermal MCCs at stage 32 by resonant confocal microscopy. Anoptical section along the MCC apical-basal axis is shown (apical up). Control MCCs (uninj. ctrl.) showed a metachronal synchronous beating pattern of cilia. Knockdown of cp110 (cp110MO) disrupted the metachronal synchronous beating pattern and caused reduced motility in MCC cilia. Movie plays at 1x speed. Related to Figure 1—figure supplement 1A–B.
Cp110 is required for unidirectional ciliary beating and ciliary motility in MCCs.
Embryos were injected with cfap20-gfp to visualize ciliary axonemes of epidermal MCCs at stage 32 by resonant confocal microscopy. Horizontal optical section through the MCC ciliary tuft is shown. Control MCCs (uninj. ctrl.) showed a unidirectional beating pattern of cilia. Knockdown of cp110 (cp110MO) caused loss of directionality and reduced motility in MCC cilia. Movie plays at 1x speed. Related to Figure 1—figure supplement 1A–B.





