Tet2 and Tet3 cooperate with B-lineage transcription factors to regulate DNA modification and chromatin accessibility
Figures

Loss of Tet2 and Tet3 in the B cell lineage results in B cell developmental blockade in vivo.
(A) Reduced bone marrow B cells (B220+ CD19+) in Tet2-/-Tet3fl/fl Mb1Cre (Tet2/3 DKO) mice. Total bone marrow cells from wild type (WT) or Tet2/3 DKO mice at eight weeks (upper) and 11 weeks (lower) were analyzed for the percentage of total B cells (CD19+B220+) by flow cytometry and the representative plots are shown. Note that the loss of B cells is apparent at 8 weeks and more pronounced at 11 weeks. (B) Tet2/3 DKO mice display a striking reduction in pre-B cells. Bone marrow pre-B cells (IgM-CD43-B220+) were analyzed by flow cytometry and representative plots are shown. The absolute numbers of pre-B cells in 8–12 week-old mice are shown in Figure 1D. (C) Reduced frequency of immature (IgM+IgD-) and mature recirculating (IgM+IgD+) B cells in DKO bone marrow. CD19+B220+ bone marrow cells from 10 week-old Tet2/3 DKO and WT mice were analyzed for cell surface IgM and IgD expression. Data shown are representative and numbers of immature and mature B cells from four mice are shown in Figure 1D. (D) Quantification of cell numbers in multiple experiments similar to those shown in Figure 1B and C. (E) B cell development in Tet2/3 DKO mice is blocked at the transition from the pro-B cell to the pre-B cell stage. Bone marrow B cells (CD19+B220+) of Tet2/3 DKO or WT mice (eight weeks) were analyzed by flow cytometry for c-kit and CD25 expression. Left panel, Representative flow cytometry plots; right panel, numbers of bone marrow B cells at pro-B and pre-B cell stages were compared between WT and Tet2/3 DKO mice (n = 4). Pro-B cells, CD19+B220+IgM-CD25-c-kit+; pre-B cells, CD19+B220+IgM-CD25+ c-kit-. (F) Tet2/3 DKO mice have reduced splenic B cells. Total splenocytes from WT or Tet2/3 DKO mice were analyzed for B220 and CD19 expression by flow cytometry. Left panel, representative flow cytometry plots. Right panel, numbers of splenic B cells in WT and Tet2/3 DKO mice (n = 3 for each age group). (G) Accumulation of B cells lacking surface IgM or IgD expression in the periphery of Tet2/3 DKO mice. B cells (CD19+B220+) were analyzed for IgM and IgD expression. Left panel, representative flow cytometry plots. Right panel, compiled data of IgM-IgD- % from multiple experiments. Tet2/3 DKO mice show a considerable age-dependent increase in the proportion of IgM-IgD- B cells lacking surface B cell receptor. (H) IgM-IgD- B cells in the Tet2/3 DKO mice expressed high level of TdT (upper panel) and preBCR (CD179a, lower panel). Ages of mice are shown on the right for (A), (B), (F), and (G). Error bars indicate standard deviations. *, p<0.05, **, p<0.01 by Student’s t test. ns, not significant.
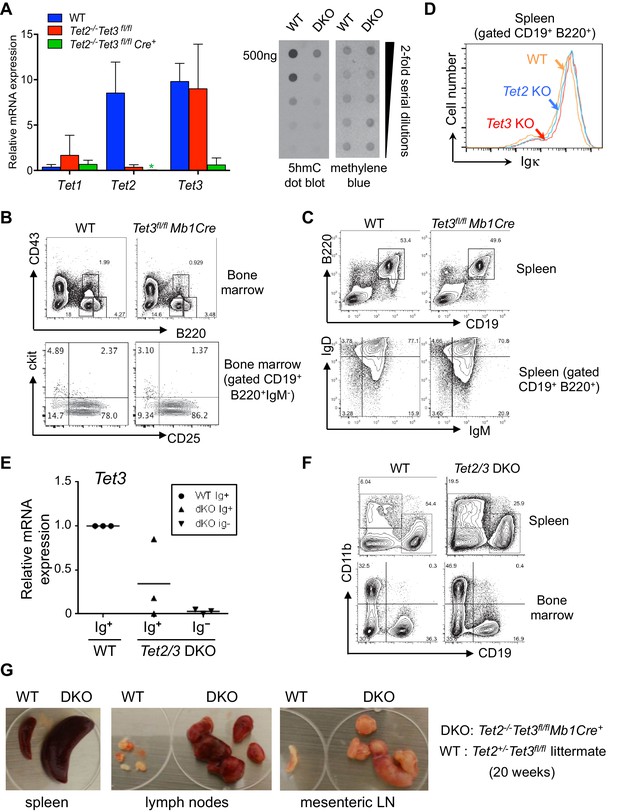
Tet2 and Tet3 are redundantly required for B cell development and BCR expression.
(A) Right, floxed Tet3 alleles are efficiently deleted in Tet2-/-Tet3fl/fl pro-B cells transduced with Cre-IRES-GFP retrovirus. Expression of Tet1, Tet2 and Tet3 mRNA in Cre+ (GFP+) WT and Tet2-/-Tet3fl/fl pro-B cells was measured by real time qRT-PCR. Left, genomic DNA was isolated from WT and Tet2/3 DKO pro-B cells and the overall level of 5hmC was detected by anti-hmC dot plot. (B) B cell development in the bone marrow is normal in the absence of Tet3. Tet3fl/fl Mb1-Cre mice (12 week-old) were analyzed. Shown are representative FACS plots for B220 and CD43 expression in the bone marrow (top panel) and c-kit and CD25 expression in the CD19+B220+IgM- bone marrow cells (lower panel). (C) Normal B cell compartment in the periphery of Tet3fl/fl Mb1-Cre mice. As in (B) splenocytes were analyzed for expression of CD19 and B220 (upper panel); IgM and IgD expression of CD19+B220+ B cells (lower panel). (D) Normal expression of cell surface Igκ in splenic B cells from Tet2-KO or Tet3-KO. Cell surface Igk was analyzed by flow cytometry as in Figure 2A. (E) Ig+ cells from Tet2/3 DKO mice have variable levels of residual Tet3 mRNA expression. Ig+ (IgM+IgD+) or Ig- (IgM-IgD-) splenic B cells (CD19+B220+) were sorted and Tet3 expression was determined by real-time qRT-PCR. (F) Compared to WT mice, Tet2/3 DKO mice have higher frequencies of myeloid cells in the spleen and bone marrow. Shown are representative flow cytometry plots of CD19 and CD11b staining. Frequencies of B cells (CD19+CD11b-) and myeloid cells (CD19-CD11b+) are indicated. (G) Formation of splenomegaly and lymphadenopathy in Tet2/3 DKO mice. Lymph nodes and spleen from a representative Tet2/3 DKO mouse and littermate control (Tet2+/- Tet3fl/+) at 20-week of age are shown.

Tet2 and Tet3 promote immunoglobulin chain expression and rearrangement in vivo.
(A) IgM-IgD- B cells in the Tet2/3 DKO mice have diminished immunoglobulin light chain expression. IgM-IgD- and IgM+IgD+ splenic B cells (CD19+B220+) were analyzed for intracellular (left panel) and cell surface (right panel) Igκ expression by flow cytometry. Data are representative of three independent experiments. (B) Tet2/3 DKO pre-B cells have reduced Igκ rearrangement. WT and Tet2/3 DKO pre-B cells (CD19+ckit-CD25+) were isolated from bone marrow by cell sorting and the Igκ rearrangement was determined by PCR amplification with Igκ intron as loading control. Representative experiment of three is shown. (C) Vκ-Jκ1 rearrangement is impaired in the Tet2/3 DKO pre-B cells. Rearrangement of Vκ-Jκ1 was quantified by real-time PCR. Data are summary of four pairs of mice and were normalized to the signal from WT. **, p<0.01 by Student’s t test.

Tet2 and Tet3 promote germline transcription of the Igκ locus and demethylation of the 3’ and distal Eκ enhancers.
(A) The Igκ locus. Diagram depicts gene segments and regulatory elements of the Igκ locus. For sequences of the 3’ and distal Eκ enhancers, see Figure 3—figure supplement 1A. (B) Flow-chart depicting generation of Tet2/3 DKO pro-B cells in vitro. Bone marrow (BM) cells from wild type (WT) or Tet2-/-Tet3fl/fl mice were cultured on OP9 stromal cells with IL-7 (10 ng/ml) for one week, then transduced with Cre-IRES-GFP retrovirus. GFP+ cells were isolated by cell sorting five days later, and cultured on OP9 cells with IL-7 for an additional week. At the end of this period, all cells expressed similar surface markers (B220+CD43+IgM-), consistent with the phenotypes of pro-B cells (Figure 3—figure supplement 2A). Tet3 deletion efficiency was >90% (not shown). (C) Acute loss of TET function results in increased DNA ‘methylation’ (5mC + 5 hmC) at the 3’ and distal Eκ enhancers. In vitro-derived pro-B cells from WT, Tet2 KO and Tet2/3 DKO mice were analyzed for the DNA modification status of 3’ and distal Eκ enhancers by bisulfite treatment of genomic DNA followed by PCR amplification and sequencing on an Illumina MiSeq platform. Error bars show the standard deviation of three independent experiments. (D) Tet2 and Tet3 are required for germline transcription of the Igκ locus and Irf4/8 in pro-B cells. Top, Diagram of the Igκ locus and the primers used to detect germline and Cκ transcription. Bottom, WT, Tet2 KO and Tet2/3 DKO pro-B cells were analyzed by real time PCR for expression of indicated genes. mRNA expression was normalized to that of Actb, and mRNA levels in WT cells were set to 1. Note the almost complete absence of germline and Cκ transcription in Tet2/3 DKO pro-B cells. Error bars represent the standard deviation of three independent experiments. **, p<0.01 in Student’s t test. (E) Loss of TET function in vivo is accompanied by increased DNA ‘methylation’ (5mC + 5 hmC) at the 3’ and distal Eκ enhancers. CD19+ cells were isolated from spleens of WT, Tet2KO (Tet2-/-Tet3fl/fl) and Tet2/3 DKO (Tet2-/-Tet3 fl/fl Mb1Cre) mice, and the DNA modification status of the 3’ and distal Eκ enhancers was determined as in (C). Error bars indicate the range of values obtained in two independent experiments.
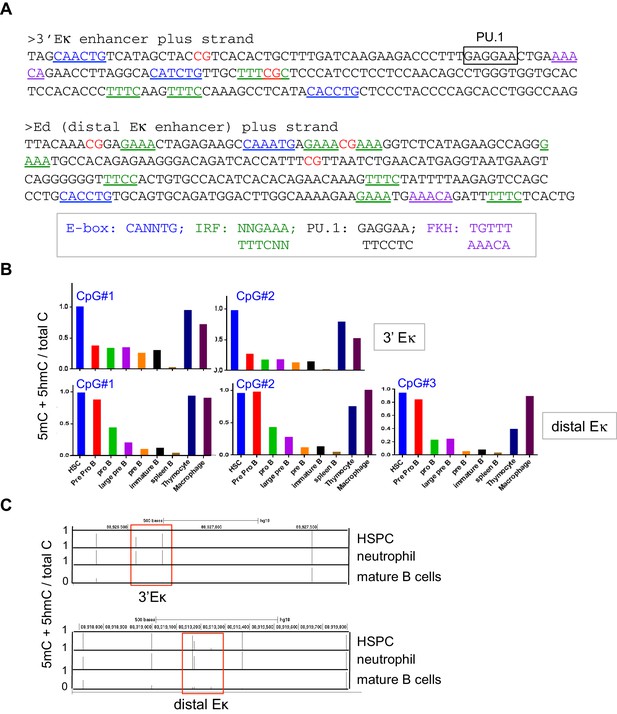
The 3’Eκ and distal Eκ enhancers undergo B cell specific-loss of cytosine modification.
(A) DNA sequences of the 3’Eκ and distal Eκ enhancers. CpG dinucleotides, consensus E-box, IRF, PU.1, and FKH motifs are indicated. (B) The 3’Eκ and distal Eκ enhancers undergo B cell-specific DNA ‘demethylation’. Control cell types and B cells at various developmental stages indicated were sorted and the DNA modification status (5mC + 5 hmC) of the 3’Eκ and distal Eκ enhancers were analyzed by bisulfite sequencing as in Figure 3C. (C) The 3’Eκ and distal Eκ enhancers undergo B cell-specific ‘demethylation’ (loss of 5mC + 5 hmC) in human B cells. Shown is the DNA modification status of human hematopoietic stem/ precursor cells (HSPC), neutrophils and B cells based on published whole-genome bisulfite sequencing data (GSE31971)(Hodges et al., 2011). Bars indicate the average fractional modification of each CpG. The regions corresponding to the 3’Eκ and distal Eκ enhancers are indicated with rectangles. Note CpG sites outside of the enhancers remain fully methylated in B cells.
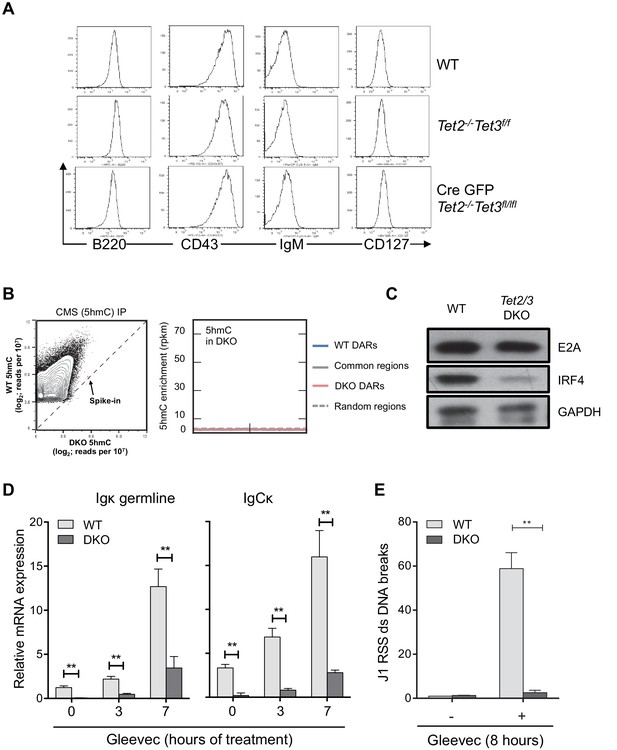
Tet2/3 regulate Igκ expression and rearrangement in BCR-Abl transformed pre-B cells.
(A) In vitro-derived WT and Tet2/3 DKO pro-B cells express similar level of pro-B surface markers. Cell-surface expression of B220, CD43, IgM, and CD127 on WT, control (Tet2-/- Tet3f/f) and DKO (Tet2-/- Tet3f/f Cre-GFP) was analyzed by FACS. (B) Right, genome-wide 5hmC distribution in WT and Tet2/3 DKO pro-B cells was mapped as described in Material and Methods. Axes represent the normalized number of reads (reads per 107) in mapped 5hmC-enriched regions combined from WT and Tet2/3 DKO. A PCR-amplified, 5hmC-containing spike-in control was used for normalization between WT and DKO (spike-in). Note that the overall 5hmC level decreased dramatically in the Tet2/3 DKO cells. Level of 5hmC in Tet2/3 DKO pro-B cells across different type of regions was plotted as in Figure 4B. (C) Decreased IRF4 protein in Tet2/3 DKO pro-B cells. The protein level of E2A and IRF4 was determined by immunoblotting with GAPDH as loading control. (D) Decreased Igκ germline transcription in DKO Abl-transformed pre-B cells. BCR-Abl transformed WT and Tet2/3 DKO pre-B cells were treated with Gleevec for 3 or 7 hr and the Igκ germline transcript expression were quantified by qRT-PCR. (E) Recombination of the Igκ locus is greatly diminished in Tet2/3 DKO pre-B cells. Ig light chain rearrangement was induced by Gleevec treatment as in (D) and the induction of DNA double-strand (ds) breaks in the Jκ1 RSS (Recombination Signal Sequence) region was determined by qRT-PCR. The results were normalized to those observed in vehicle (DMSO)-treated cells. The data summarize two independent experiments. **, p<0.01 in student’s t test.
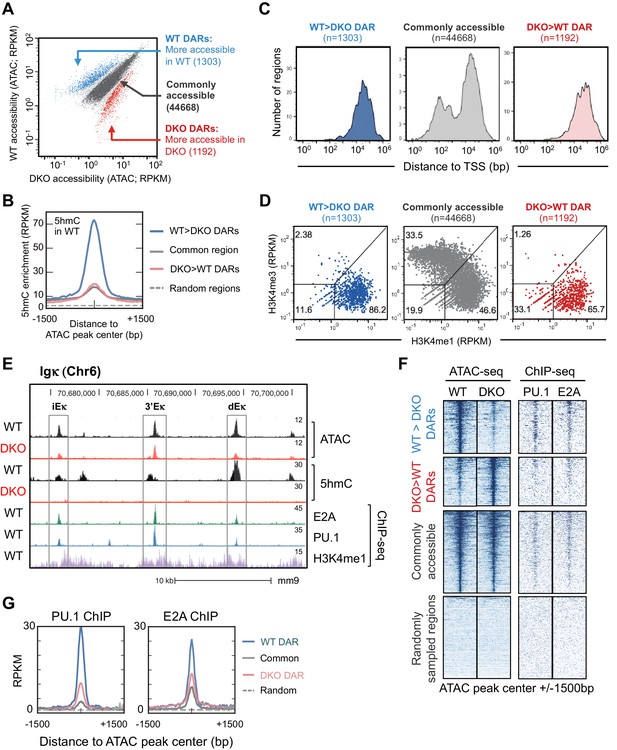
Tet2 and Tet3 regulate genome-wide enhancer accessibility in B cells.
(A) ATAC-seq identifies TET-regulated accessible regions in the genome. WT and Tet2/3 DKO pro-B cells were generated as described in Figure 3 and used to prepare ATAC-seq libraries with two replicates for each genotype. Accessible regions were identified by MACS2 and reads enrichment at each region was complied by MEDIPS and is shown as mean Reads Per Kilobase per Million mapped reads (RPKM) from two replicates, with each dot represent one accessible region (total region = 55999). Differential accessible regions were identified by MEDIPS with an adjusted p value ≤ 0.1. Blue dots indicate regions with higher accessibility in WT, or WT differential accessible regions (WT DAR; n = 1303); red dots indicate regions with higher accessibility in DKO, or DKO DAR (n = 1192); grey dots indicate regions with no statistically significant (p>0.1) with darker grey indicating regions with less than 4-fold difference (n = 44668) which was used for subsequent analysis as commonly accessible regions. (B) 5hmC is highly enriched in WT DARs. Genome-wide distribution of 5hmC in WT pro-B cells was assessed by CMS-IP (Figure 3—figure supplement 2B) and the enrichment of 5hmC across indicated accessible regions (centered and extended ± 1500 bp) was plotted with y-axis showing the mean RPKM. Random genomic regions (n = 44668) were shown as reference for background level of 5hmC. (C) TET-regulated WT DARs are primarily distal to transcription start sites (TSS). Distance between regions from (A) and closest TSS was plotted on X-axis (log 10) with a number of regions on Y-axis. (D) Tet2 and Tet3 regulate the accessibility of enhancers in pro-B cells. The enrichment of H3K4me1 and H3K4me3 (Lin et al., 2010) at regions surrounding ATAC-seq peaks (±250 bp) were analyzed and plotted, with X-axis indicating H3K4me1 and Y-axis indicating H3K4me3. ATAC-seq peaks were classified into promoters (H3K4me1low, H3K4me3high), enhancers (H3K4me1high, H3K4me3low), or other regions (H3K4me1low, H3K4me3low), which may include insulators, silencers and locus control regions. The frequencies of each class of ATAC-seq peaks are indicated. (E–G) TET-regulated regions are enriched for E2A and PU.1 binding sites. (E) Genome browser view of the 3’ end at the Igκ locus. From top to bottom are tracks for ATAC-seq and 5hmC/CMS-IP tracks of WT or Tet2/3 DKO pro-B cells (combined from two replicates), followed by E2A, PU.1, and H3K4me1 tracks from WT pro-B cells. Rectangles show the locations of the intronic (iEκ), 3’ (3’Eκ) and distal Eκ (dEκ) enhancers. (F) Left panel shows the ATAC-seq signal from WT (1st column) and DKO (second column) across indicated type of regions, with each horizontal line representing one region/locus. Note that the commonly accessible and randomly sampled regions are compressed compared to WT and DKO DARs in order to accommodate all regions. Right panel shows PU.1 and E2A ChIP-seq signal from published datasets across all regions. Randomly sampled regions are included for comparison. (G) Mean RPKM signals of PU.1 (left) and E2A (right) were shown as a histogram. Note that the WT DARs (blue) have the stronger enrichment of both transcription factors compared to other regions. Analysis of additional transcription factors can be found in Figure 4—figure supplement 2.
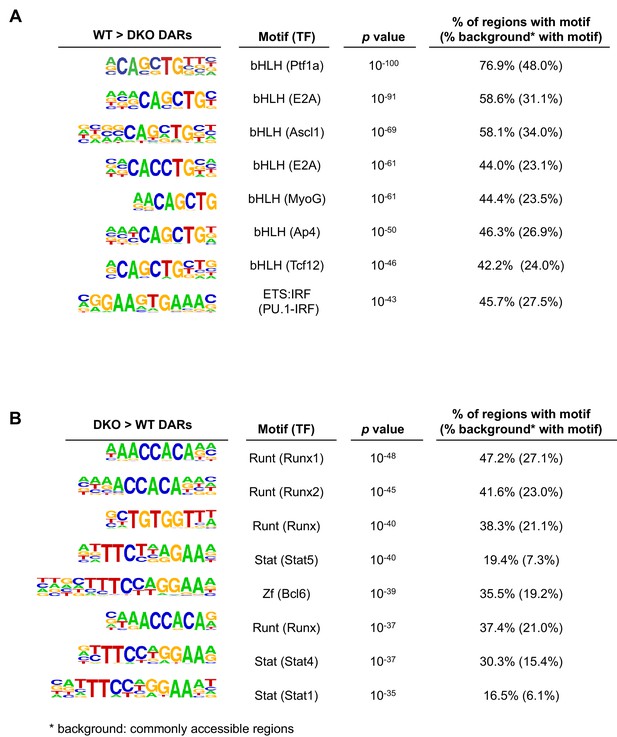
Identification of sequence motifs enriched in differential accessible regions.
TET-regulated WT > DKO accessible regions (WT>DKO DARs; more accessible in WT than in Tet2/3 DKO pro-B cells) (A) and DKO > WT accessible regions (DKO>WT DARs) (B) were analyzed for enrichment of transcription factor motifs with HOMER with commonly accessible regions (similar accessibility between WT and DKO cells) as background. Top eight motifs from each analysis are shown.
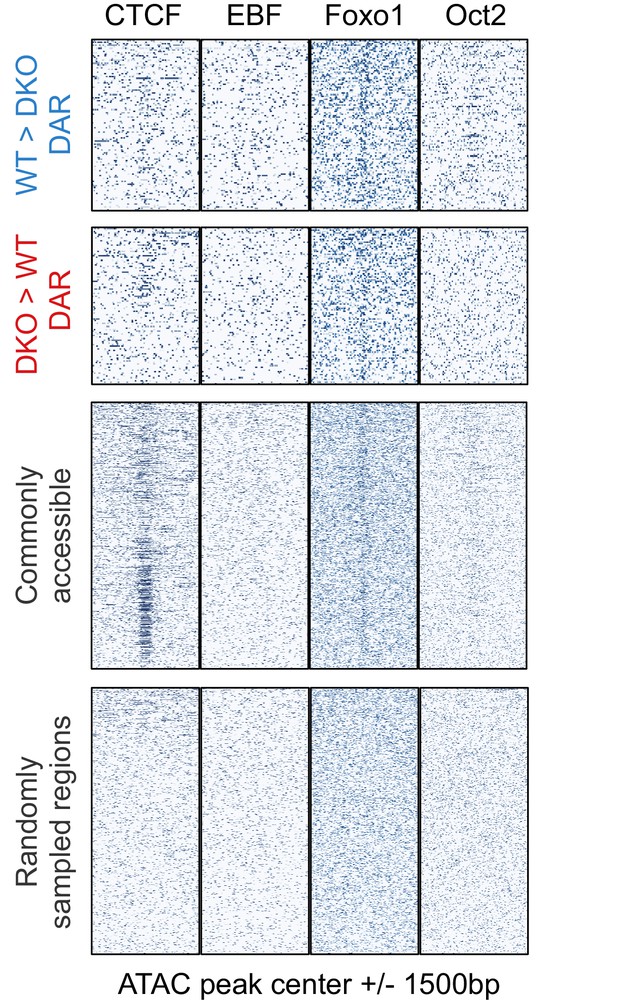
Binding pattern of additional B-cell-specific and general transcription factors at accessible regions.
ChIP-seq signals (Heinz et al., 2010) of CTCF, EBF, Foxo1, and Oct2 (in mature B cells) are plotted for the following regions as in Figure 4F: TET-regulated WT>DKO differential accessible regions (DAR; less accessible in Tet2/3 DKO cells than in WT), DKO>WT DAR (more accessible in Tet2/3 DKO than in WT), commonly accessible regions, and randomly sampled regions.
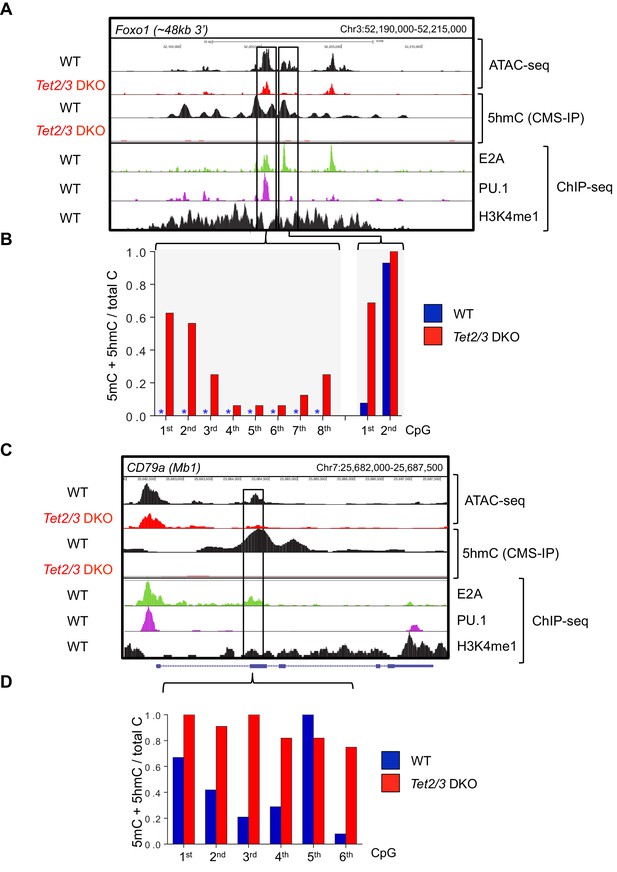
Tet2 and Tet3 regulate the accessibility and demethylation of enhancers associated with genes critical for B cell development.
Genome browser views of the Foxo1 (A) and CD79α (C) loci in WT and Tet2/3 DKO pro-B cells. (A, C) From top to bottom are ATAC-seq peaks and 5hmC (CMS) in WT and Tet2/3 DKO pro-B cell, followed by E2A, PU.1, and H3K4me1 ChIP-seq signal from WT pro-B. (B, D) Bisulfite sequencing analysis of the indicated enhancers at the respective locus. Asterisks indicate absence of methylation.
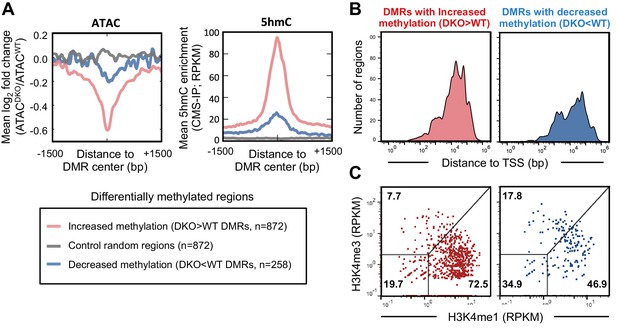
TET proteins demethylate and modulate the accessibility of enhancers.
DNA isolated from WT and Tet2/3 DKO pro-B cells (two replicates each) were bisulfite-treated and sequenced to analyze the genome-wide DNA cytosine methylation. Differential methylation regions were analyzed as described in Materials and Methods, and 872 and 258 regions were found to have more methylation in DKO (‘DKO > WT DMRs' in red) and WT (DKO<WT DMRs’ in blue), respectively. (A) Regions with increased methylation in Tet2/3 DKO are highly enriched in 5hmC and more accessible in WT compared to Tet2/3 DKO. Left, the log2 ratio between ATACWT and ATACDKO was calculated for the indicated regions (bin size = 10 bp) and the means were plotted. Right, ‘DKO > WT methylation’ regions were marked with 5hmC in WT pro-B cells. The mean RPKM values for 5hmC enrichment (detected by CMS-IP) were plotted for each set of regions. (B–C) Differentially methylated regions bear the features of enhancers. (B) Distance of differential methylated sites to the closest transcription start sites (TSSs) was plotted as histogram as in Figure 4C. (C) Relative enrichment of H3K4me1 and H3K4me3 at the differential methylated regions was plotted as in Figure 4D.
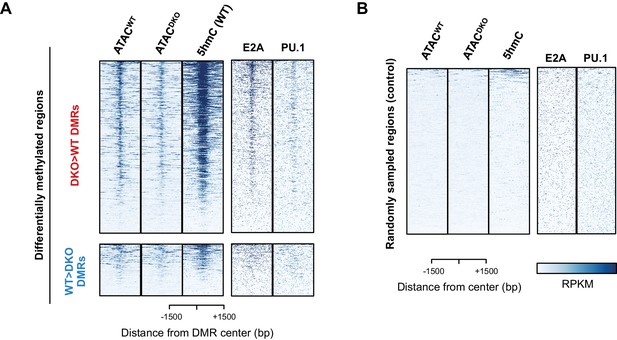
TET proteins demethylate and modulate the accessibility.
(A–B) Differentially methylated regions (DMRs) were analyzed as described in Materials and Methods, and 827 and 258 regions were found to have more methylation in DKO (upper panel in (A); ‘DKO > WT DMRs’ in red) and WT (lower panel in (A); ‘WT > DKO DMRs’ in blue), respectively. The first two columns show the ATAC-seq signal from WT (ATACWT) and Tet2/3 DKO (ATACDKO) for indicated regions (center of DMR ± 1500 bp), followed by the 5hmC signal from WT pro-B cells on the third column. ChIP-seq for E2A and PU.1 are shown on the right. Randomly sampled regions were used to show the background signals in (B).
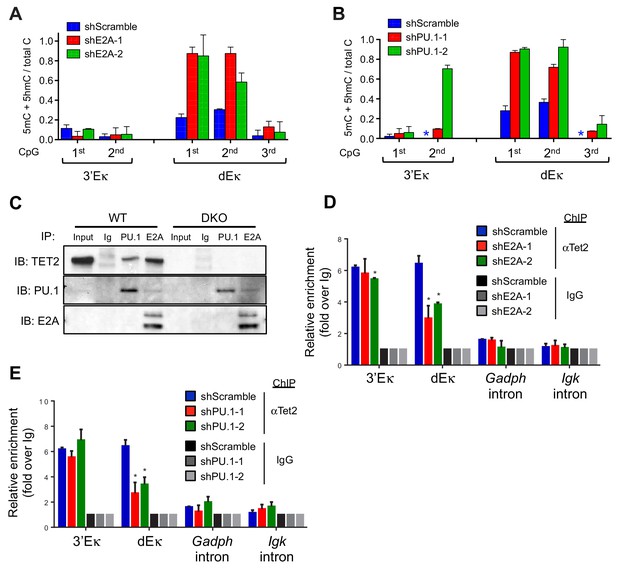
Lineage-specific transcription factors cooperate with TET protein in inducing Igκ enhancer demethylation.
(A) Depletion of E2A increases DNA modification (5mC + 5 hmC) at the distal Eκ enhancer. WT BCR-Abl-transformed pre-B cells were transduced with two independent shRNAs against E2A or scrambled shRNA as a control. Methylation of 3’Eκ and dEκ was determined by bisulfite sequencing in the control or E2A-depleted cells. E2A knockdown is verified by immunoblotting (Figure 6—figure supplement 1A). Data are the summary of two independent experiments. Error bars show the range of duplicates. (B) Depletion of PU.1 increases DNA modification (5mC + 5 hmC) at the distal Eκ enhancer. As in (A), methylation of 3’Eκ and dEκ enhancers was analyzed by bisulfite sequencing after PU.1 knockdown. PU.1 knockdown is verified by immunoblotting (Figure 6—figure supplement 1B). Error bars show the range of duplicates. (C) Tet2 directly interacts with PU.1 and E2A. E2A and PU.1 were immunoprecipitated from WT or Tet2/3 DKO BCR-Abl pre-B cell nuclear extract in the presence of ethidium bromide and benzonase to prevent indirect 'interaction' via DNA. Co-immunoprecipitated proteins were probed with anti-Tet2. Input loaded was 2.5%. Note that a different secondary antibody was used for PU.1 and E2A to avoid the interference of IgH and IgL and thus weaker signal. (D–E) E2A and PU.1 facilitate the binding of Tet2 to dEk. E2A (D) and PU.1 (E) were depleted by shRNAs as in (A) and (B) and the association of Tet2 to Igκ enhancers or control regions (Gadph and Igκ introns) were assessed by ChIP-qPCR. Data are representative for at least two experiments.
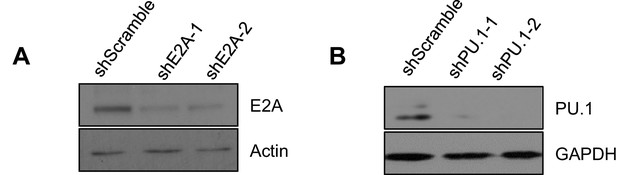
Efficient knockdown of E2A and PU.1.
WT Abl-transformed pre-B cells were transduced with two individual shRNAs targeting E2A (A) and PU.1 (B) and the respective protein level was detected by immunoblotting. Actin (A) and GAPDH (B) were used as loading controls.
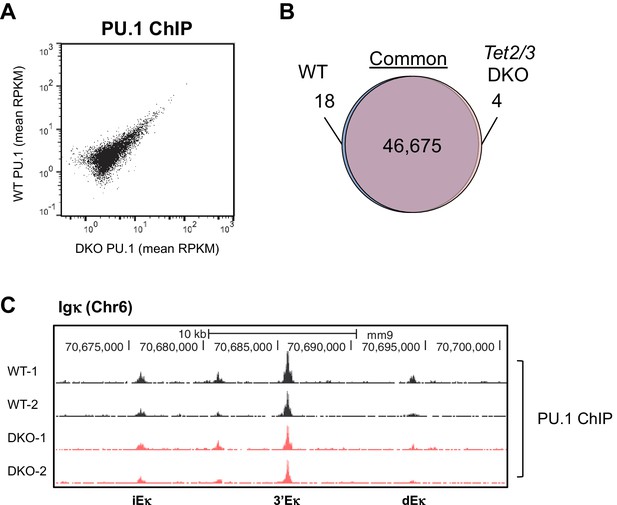
Tet2/3-deficiency has limited effect on genome-wide PU.1 binding.
PU.1 binding patterns in cultured WT and Tet2/3 DKO pro-B cells were analyzed by ChIP-seq, with two biological replicates for each genotype. (A) Similar global PU.1 binding in WT and Tet2/3 DKO cells. Reads were to reference genome (mm9) by Bowtie2 and the peaks were called with MACS2 (-q 0.05). All peaks identified from all samples were merged to generate a master peak set, which were used as regions of interest for subsequent analysis by MEDIPS. Mean signals (in RPKM) for each peak was plotted, with those from Tet2/3 DKO on X-axis and WT on Y-axis. (B) Venn diagram showing the overlapping between WT and Tet2/3 DKO PU.1 peaks. Differential binding peaks between WT and Tet2/3 DKO were selected with MEDIPS based on adjusted p≤0.1. Note that > 99.9% of the peaks are not significantly different between WT and Tet2/3 DKO pro-B cells. Details of unique regions are shown in Supplementary file 2. (C) Genome browser view of PU.1 ChIP-seq tracks from two replicates each for WT (black) and Tet2/3 DKO (red) at Igκ enhancers as in Figure 4E.
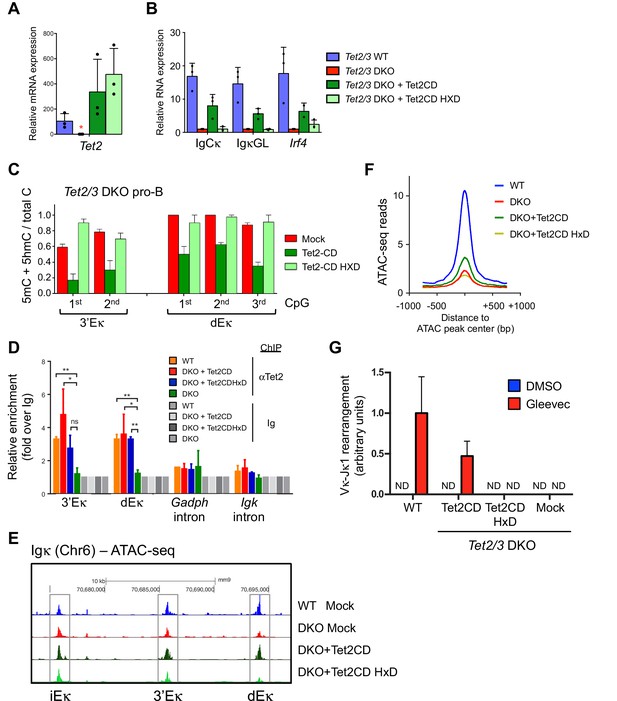
Tet2CD rescues Igκ expression, promotes demethylation and accessibility of 3’Eκ and distal Eκ enhancers.
(A–B) The enzymatic activity of Tet2 is required to promote Irf4 expression and Igκ germline transcription. BCR-Abl-transformed Tet2/3 DKO pre-B cells were transduced with retrovirus expressing Tet2CD, the corresponding Tet2CD HxD catalytic inactive mutant or empty vector (mock) together with a blasticidin resistance gene. Seven days post blasticidin selection, expression of Tet2 (A), Cκ, Igκ germline transcripts and Irf4 was determined by real time PCR (B). Error bars represent the standard deviation of three independent experiments. *, p<0.01 in Student’s t test. (C) The enzymatic activity of Tet2 is required to promote ‘demethylation’ (loss of 5mC + 5 hmC) at Igκ enhancers. BCR-Abl-transformed Tet2/3 DKO pre-B cells were transduced as in (B), and the DNA modification status of the 3’ and distal Eκ enhancers was analyzed by bisulfite sequencing. Error bars indicate the range of values obtained in two independent experiments. (D) Tet2 associates with the Igk enhancers. WT, Tet2/3 DKO, and DKO transduced with Tet2CD or Tet2CD HxD Abl-transformed pre-B cells were used as input and Tet2 binding to indicated regions was detected by ChIP-qPCR. The signal was normalized to corresponding samples immunoprecipitated with Ig. (E) Tet2CD restores chromatin accessibility at the Igκ enhancers in Tet2/3 DKO cells. Tet2/3 DKO pro-B cells were transduced with retrovirus containing Tet2CD-IRES-Thy1.1 (Tet2CD), Tet2CD HxD mutant-IRES-Thy1.1 (Tet2CD HxD) or empty vector (mock). Thy1.1+ cells were sorted and chromatin accessibility was analyzed through ATAC-seq. (F) Tet2 requires its enzymatic activity in promoting chromatin accessibility. TET-regulated accessible regions (WT>DKO DARs) were identified as in Figure 4A, and chromatin accessibility of these regions were plotted in WT pro-B cells, or Tet2/3 DKO pro-B cells reconstituted with Tet2CD, Tet2CD HxD or empty vector (mock). Histogram shown is distribution of ATAC-seq reads (normalized to 10 million reads depth) over 750 bp downstream or upstream of the peak center. Verification of Tet2 expression by immunoloblotting is shown in Figure 8A. (G) Tet2CD restores VκJ recombination in Tet2/3 DKO pre-B cells. Abl-transformed pre-B cells transduced with Tet2CD or Tet2CD HxD were treated with Gleevec to induce VκJ rearrangement and analyzed as in Figure 2C by ligation-mediated-PCR.

TET regulates expression of IRF4.
(A) Immunoblotting for Tet2 and IRF4 in Tet2/3 DKO BCR-Abl cells transduced with Tet2CD or Tet2CD HxD mutant. (B) Reconstitution with Irf4 only marginally rescued Igκ expression in the Tet2/3 DKO cells. Tet2/3 DKO cells were transduced with retrovirus expressing IRF4 and IgCκ and IgκGL transcription was analyzed by qRT-PCR. (C) Re-expression of IRF4 has no effect on enhancer methylation in the absence of Tet2/3. Enhancer methylation status of cells form (B) was analyzed by bisulfite sequencing. (D) Working model of TET-mediated regulation of the Igκ locus. During early B cell development, the pioneer transcription factor PU.1 binds at various locations including the Igκ enhancers (e.g. 3’Eκ and dEκ shown here; left). Subsequently, presumably after IgH rearrangement, PU.1 and potentially E2A recruit TET proteins, facilitating the deposition of 5hmC and DNA demethylation. These TET-dependent activities increase enhancer accessibility, likely resulting in increased binding of additional transcription factors (middle). In the absence of TET proteins, the enhancers remain methylated and are less accessible for transcription factors (right). In addition, TET proteins regulate the expression of IRF4, a transcription factor important for the induction of Igκ rearrangement (top).
Additional files
-
Supplementary file 1
Primer sequences.
- https://doi.org/10.7554/eLife.18290.019
-
Supplementary file 2
Unique regions from PU.1 ChIP.
- https://doi.org/10.7554/eLife.18290.020





