Phenotypic outcomes in Mouse and Human Foxc1 dependent Dandy-Walker cerebellar malformation suggest shared mechanisms
Figures
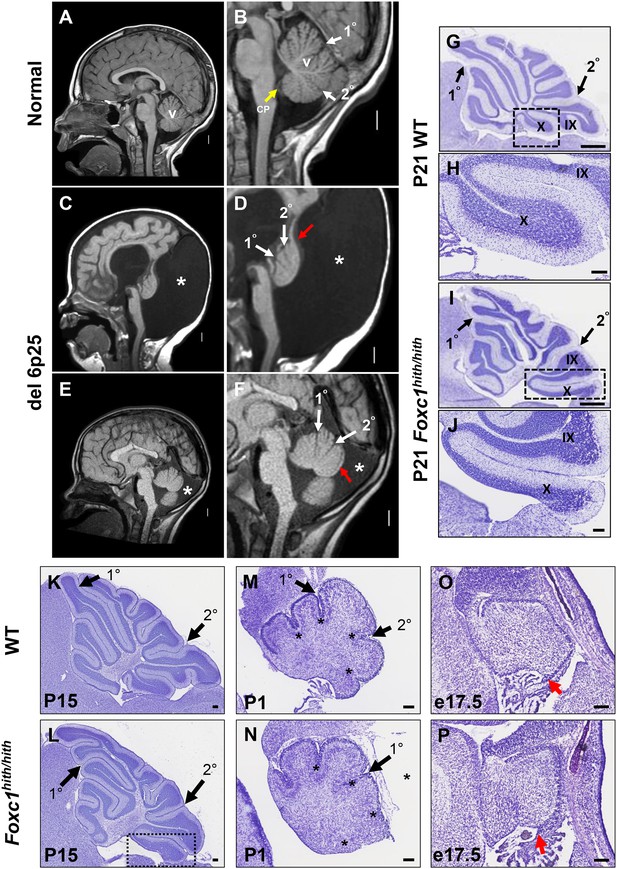
Similarities of human DWM and mouse Foxc1hith/hith posterior folial abnormalities.
(A–F) T1-weighted midsagittal magnetic resonance images in the postnatal control subject (A,B) and two subjects with del chr 6p25.3 CNVs that include FOXC1 or intragenic mutations of FOXC1 diagnosed with Dandy Walker Malformation (Aldinger et al., 2009) (C–F). The midline cerebellar vermis (v) and choroid plexus (CP) are marked only in the controls. Asterisks (*) indicate an enlarged posterior fossa in DW cases. The white arrowheads mark the 10 and 20 fissures, while the red arrowhead indicates upward rotation of the cerebellar vermis and abnormal posterior DW tail. Sagittal sections of P21 cerebellar vermis from wild-type (G,H, K, M, O) and Foxc1hith/hith (I,J, L, N, P) mice. The Foxc1hith/hith cerebellum is characterized by the presence of a partially formed posterior lobule X (I,L box; J). The stereotypical wild-type cerebellum foliation pattern is disrupted in Foxc1hith/hith mutants. Primary and secondary fissures are noted (black arrowheads). Four cardinal fissures (black asterisks) divide wild-type postnatal cerebellar vermis into five cardinal lobes. Foxc1hith/hith mice exhibit an excess of granule cell progenitors (GCPs) in the e17.5 RL (P, red arrowhead). Scale bars = 100 µm (H, J, K–P) and 500 µm (G,I).
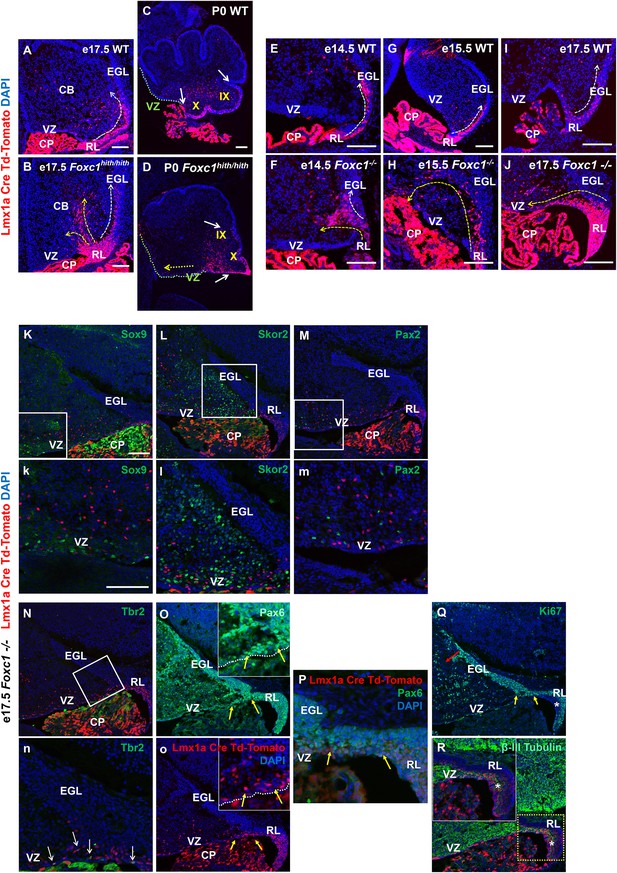
Null and Hypomorphic Foxc1 mutations caused posterior cerebellar foliation defects due to mismigration of cells destined to form the posterior vermis.
(A–J) Lineage analysis of the Lmx1a-cre+ cells in the wild-type mice showed tdTomato expression limited to the RL, EGL and presumptive IGL. Postnatally, fate-mapped cells populated the posterior vermis but did not abut the 2o fissure (C, white arrows). In the wild-type embryonic cerebellum, these cells were present underneath the EGL directly underneath the pial surface (A, E, G, I; white arrow). In Foxc1hith/hith Lmx1a-cre tdTomato mice, cells migrated out of the RL in multiple ectopic streams (B, yellow arrows). Postnatally, in the Foxc1hith/hith mutant cerebellum, ectopic tdTomato+ cells were present along the ventricular surface and the inner cerebellar core (D, yellow arrows). In Foxc1-/- mice (F,H,J), aberrantly migrating Lmx1a-cre tdTomato+ cells were evident by e14.5 in the core (F) and found in the VZ by e15.5 (H, yellow arrow), with an extensive VZ surface presence by e17.5 (J; yellow arrow). Additionally, at e17.5, a large number of fate-mapped mutant cells were abnormally retained in an enlarged RL (J). None of the mutant internal tdTomato+ cells were Sox9+ (K,k), Skor2+ (L,l) or Pax2+ (M,m), and thus had not undergone a VZ lineage fate-switch. A subset of the fate-mapped cells were Tbr2+ (N,n, arrows), as expected of RL-derived unipolar brush cells. All tdTomato+ cells were Pax6+ (O–P). This indicated that they retained their RL origin despite aberrant migration. A subset of the Pax6+ cells is Ki67+ (O, o, Q; yellow arrows) indicating that they retain their ability to divide, while some tdTomato+ cells in the RL (asterisk) are β-III Tubulin+ (R) and Ki67- indicating that they may have differentiated precociously. Scale Bar = 100 µm (A–D, K–Q), 50 µm (E–J).
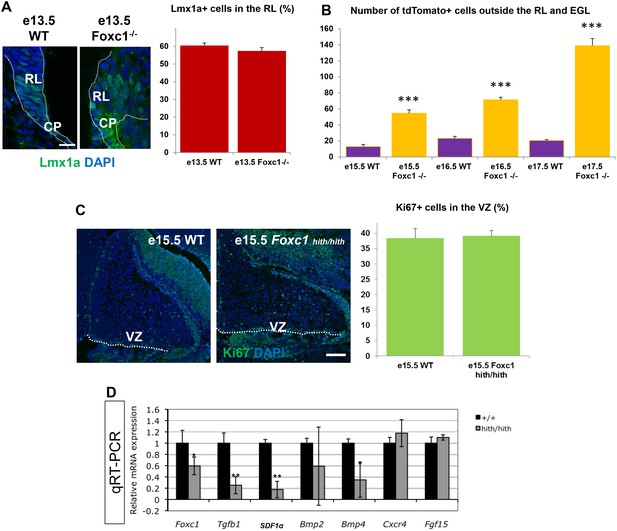
A significant number of ectopic tdTomato+ cells are found in the Foxc1 mutant cerebellum.
(A) Quantification of Lmx1a+ cells in the RL of WT and Foxc1 null mutants indicate that there is no difference in Lmx1a expression. p=0.17. Scale bar = 20 µm (B) Quantification of tdTomato+ cells present outside the EGL and RL area in WT and Foxc1 null mutants indicate that there is a significantly higher number of tdtomato+ cells present in the mutant, many of which are ectopic in nature. ***p<0.005 (C) Quantification of Ki67+ cells in the VZ of WT and Foxc1hith/hith mutants indicate that there is no difference in proliferation. p=0.4. Scale bar = 100 µm (D) Mid-hindbrain expression of Foxc1 targets in e12.5 wild-type (black) and Foxc1hith/hith (grey) littermate embryos, assayed by qRT-PCR. Foxc1 reduction decreases hindbrain mesenchyme expressed genes (Tgfb1, SDF1α, Bmp2 and Bmp4), but not neural tube expressed genes (Fgf15 and Cxcr4). *p<0.05, **p<0.0001.
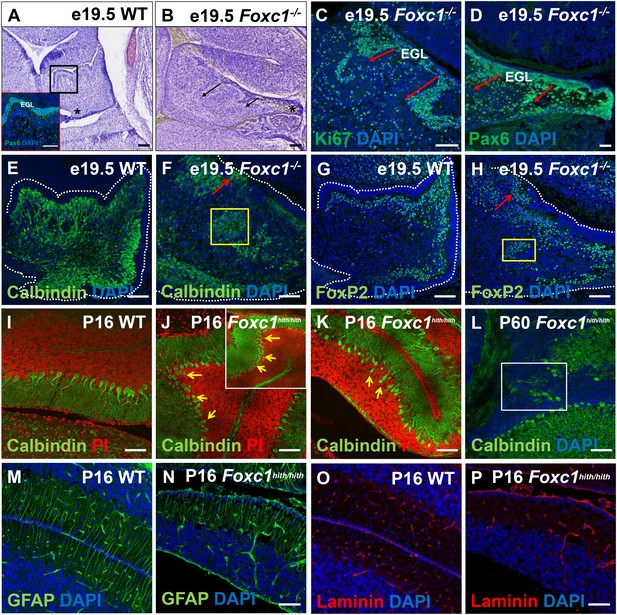
Ectopic populations of granule cell progenitors and Purkinje cells are found in both the Foxc1-/- and Foxc1hith/hith mutants.
(A–H) Sagittal sections of the e19.5 wild-type (A,E,G) and Foxc1-/- (B–D, F,H) cerebellum stained with Cresyl violet (A,B), Ki67 (C) and Pax6 (A; inset and D), showed the presence of ectopic GCPs in the Foxc1-/- cerebellum migrating precociously into the IGL from the pial surface (C,D; arrows). Multiple ectopic Purkinje cells were also found in the Foxc1-/- cerebellum (F,H, arrows, yellow box) as indicated by Calbindin (E,F) and Foxp2 staining (G,H). In P16 wild-type mice, Calbindin staining (I–L) demarked a monolayer of Purkinje cells (I). In P16 and P60 Foxc1hith/hith mice, Purkinje cells were disorganized, arranged in multiple layers (J,K; arrows), and ectopically embedded in the IGL (L, white box). Sagittal sections of the WT (M,O) and Foxc1hith/hith cerebellum (N,P) stained for GFAP (M,N) and Laminin (O,P) indicated that Bergmann glial fibers and the pial surface were structurally normal in the Foxc1hith/hith mutant cerebellum. Scale Bar = 100 µm.
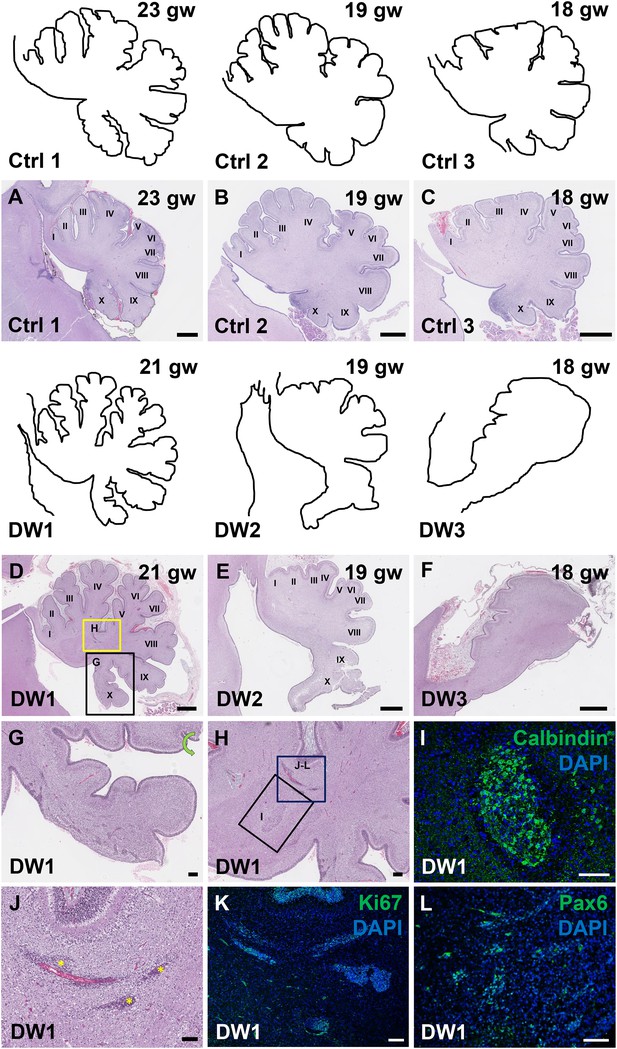
Defects in cerebellar foliation and histogenesis were observed in human DWM cases with deletions in chromosome 6p25.
(A–I) Hematoxylin and Eosin stained midsagittal sections through the fetal cerebellar vermis in normal (A–C) and del chr 6p25 samples (D–F). Ages are indicated in gestational weeks (gw). Cerebellar outlines are provided for clarity with higher magnification locations indicated. The Xth lobule of the posterior vermis in DW1 (D, black box; G) was only partially formed, similar to the Foxc1hith/hith cerebellum while in (E–F), the posterior vermis was severely dysplastic in DW2 and DW3. The del chr 6p25 cerebella also had ectopic Calbindin+ Purkinje cells (H, box and I) and ectopic Ki67+ Pax6+ GCPs (H, box J–L). Scale bar = 1 mm (A–F), 200 µm (G) and 100 µm (H–L).
-
Figure 3—source data 1
List of control and Dandy-Walker malformation cases listed in the study.
- https://doi.org/10.7554/eLife.20898.007
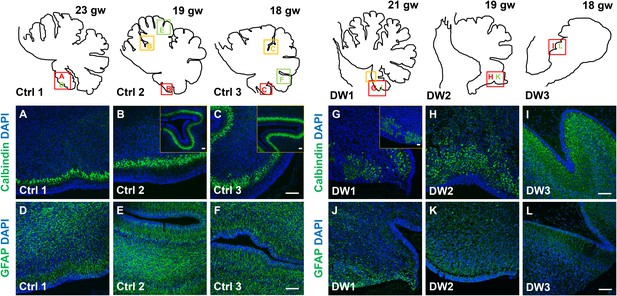
Defects in Purkinje cell alignment and Bergmann glial morphology were observed in the cerebellum of fetuses with chr 6p25 mutations.
(A–L) Midsagittal sections through normal (A-F) and del chr 6p25 (G–L) fetal cerebellar vermis stained for Calbindin (A–C, G–I) and GFAP (D–F, J–L). Cerebellar outlines are provided for clarity. Colored boxes show locations of panels. Ages are indicated in gestational weeks (gw). Purkinje cells during normal cerebellar development were arranged in a distinct multilayered band beneath the molecular layer throughout the cerebellum from 18–23 gw (A–C, insets). In all del chr 6p25 cases, Purkinje cells were ectopically broadly distributed in the forming cerebellar cortex (G–I). Bergmann glial fibers extended from the PC layer to the EGL in the normal cerebellum (D–F). These fibers were sparse and highly dysmorphic in the del chr 6p25 cases (J–L). Scale bar = 100 µm.
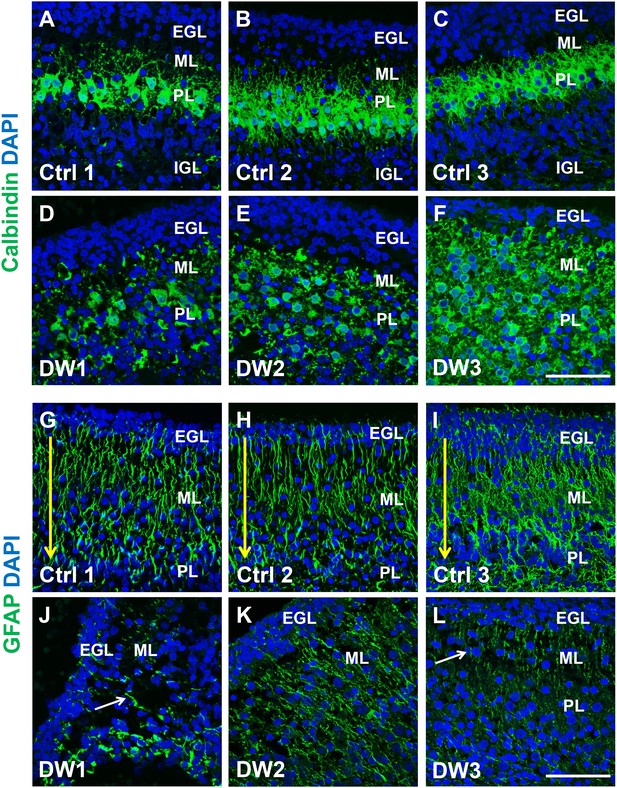
Purkinje cell and Bergmann glial fiber morphologies were disrupted in human fetal del chr 6p25 cases.
(A–L) Midsagittal sections through the fetal cerebellar vermis in control fetuses (A–C, G–I) and del chr 6p25 cases (D–F, J–L) stained for Calbindin (A–F; green) and GFAP (G-–L; green). In all control cases, Purkinje cells formed a compact multilayered band beneath the molecular layer, with nascent dendrites projecting into the molecular layer (A–C). In all del chr 6p25 cases, the Purkinje cells were dispersed as a highly disorganized multi-layer zone. Additionally, several cells were ectopically located in the molecular layer (D–F). In control cases, Bergmann glial fibers extended from the EGL to the IGL (G-–I; arrow). There were fewer fibers in all del chr 6p25 cases and their morphology was severely disrupted (J–L, arrows). Scale bar = 50 µm.
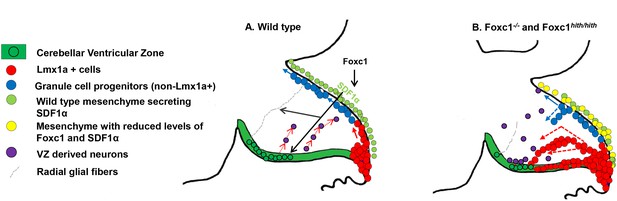
Summary of Foxc1-dependent molecular and cellular mechanisms contributing to del chr 6p25 cerebellar phenotypes.
(A) Schematic of a paramedial sagittal section of the embryonic mouse cerebellum. In the wild-type cerebellum, mesenchymal Foxc1 controls the expression of chemokine SDF1α which binds to its receptor Cxcr4 which is strongly expressed in the RL, EGL, and VZ. SDF1α functions as a chemoattractant to Lmx1a+ (red) and Lmx1a- (blue) GCPs exiting the RL to form the EGL, ensuring that these progenitors exit the RL and remain confined to the EGL underneath the pial surface. SDF1α also controls the migration of cells out of the VZ, acting as a chemoattractant. It is also required for the maintenance of radial glial fibers, which act as scaffolds for this migration. (B) In the Foxc1-/- and Foxc1hith/hith mice, deletion of Foxc1 leads to a significant downregulation of mesenchymal SDF1α by e12.5. This reduction results in excessive retention of posterior-fated cells in the RL and ectopic migration of cells out of the RL (red arrows) and precocious migration of GCPs from the EGL into the cerebellar anlage (blue arrows). Proliferation, migration, and VZ-derived neurons and radial glia are also negatively affected.





