Peripherally administered orexin improves survival of mice with endotoxin shock
Figures
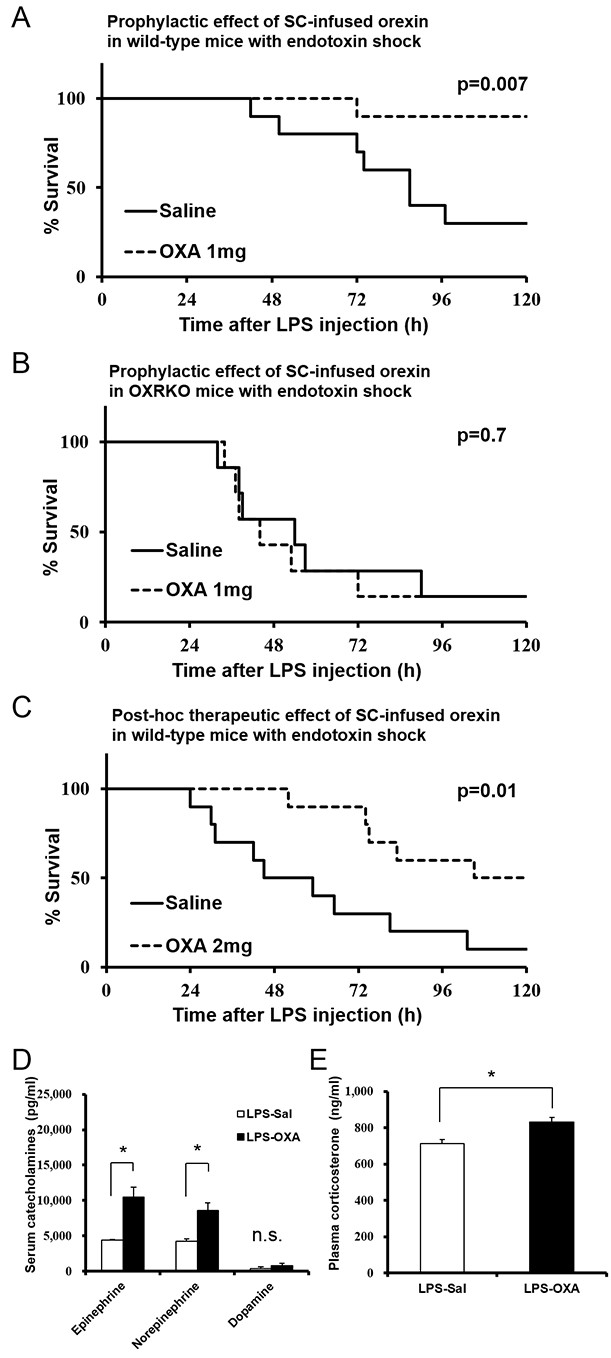
Effects of peripherally administered orexin-A (OXA) on survival in mice with endotoxin shock.
(A) Kaplan-Meier survival curves of wild-type mice subcutaneously (SC) administered with saline or orexin-A (OXA; 1 mg/mouse/24 hr) 30 min before lipopolysaccharide (LPS; 10 mg/kg) injection (each group n = 10). (B) Kaplan-Meier survival curves of Hcrtr1-/-;Hcrtr2-/- (OXRKO) mice SC-administered with saline or OXA (1 mg/mouse/24 hr) 30 min before LPS injection (each group n = 7). (C) Kaplan-Meier survival curves of wild-type mice SC-administered with saline or OXA (2 mg/mouse/24 hr) 30 min after LPS injection (each group n = 10). (D, E) Effect of OXA treatment on the levels of catecholamines (D) in the serum and corticosterone (E) in the plasma from LPS-injected mice, compared to saline treatment (each group n = 3–5, *p<0.05). Data are presented as mean±s.e.m. Statistical significance assessed by Mantel Cox log-rank test (A–C) and unpaired t-test (D, E). Data are replicated in at least three independent experiments. n.s: not significant.
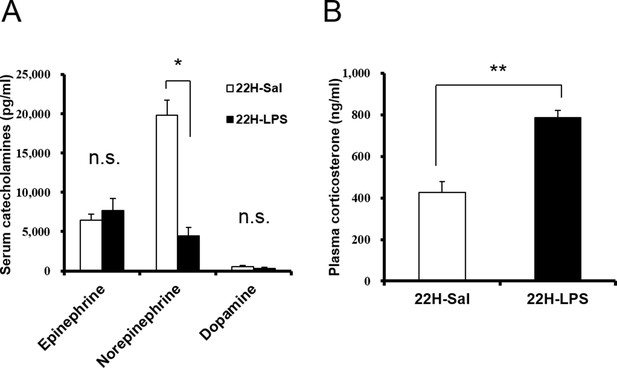
Serum catecholamines (A) and plasma corticosterone (B) levels at 22 hr after LPS injection.
Norepinephrine level in the serum decreased and corticosterone level in the plasma increased at 22 hr after LPS injection (22H-LPS), compared to saline injection (22H-Sal) (each group n = 3–5, *p<0.05, **p<0.01).
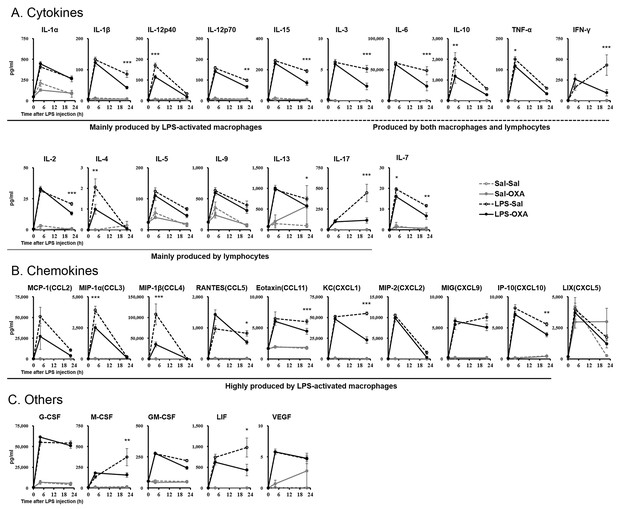
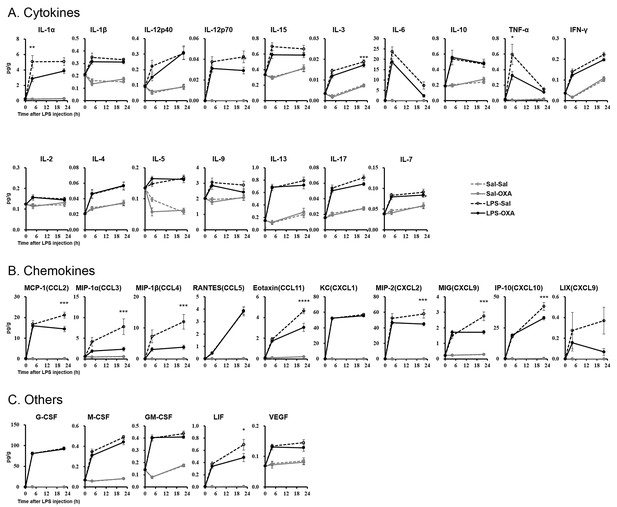
Effects of peripherally administered OXA on cytokine production in mice with endotoxin shock.
Effects of OXA treatment on the levels of 32 cytokines in serum at 4 hr and 22 hr after injection of LPS (LPS-OXA) or saline (Sal-OXA), compared to saline treatment (LPS-Sal, Sal-Sal) (each group n = 8, *p<0.05, **p<0.01, ***p<0.001). OXA (1 mg/mouse/24 hr) started to be SC-administered at 30 min before LPS injection. Statistical significance assessed by 2-way ANOVA coupled with the Bonferroni’s test. Data are replicated in at least three independent experiments.
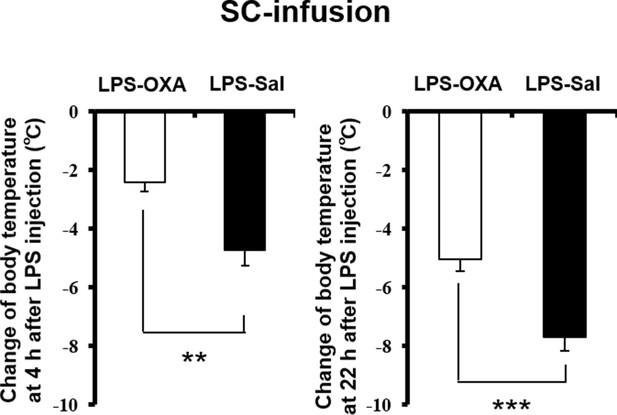
Effects of SC-infused OXA on body temperature at 4 hr or at 22 hr after LPS injection.
The changes of body temperature at 4 hr or 22 hr after LPS injection by SC administration of OXA (1 mg/mouse/24 hr, LPS-OXA), compared to saline administration (LPS-Sal) (each group n = 8, ***p<0.001, **p<0.01).
Effects of SC-infused OXA on cytokine production in brain at 4 hr or at 22 hr after LPS injection.
LPS increased most cytokines in all samples assayed. SC administration of OXA (1 mg/mouse/24 hr, LPS-OXA) for 4 hr or 22 hr decreased several cytokine levels in the brain from LPS-injected mice, compared to saline SC administration (LPS-Sal) (each group n = 8, *p<0.05, **p<0.01, ***p<0.001).
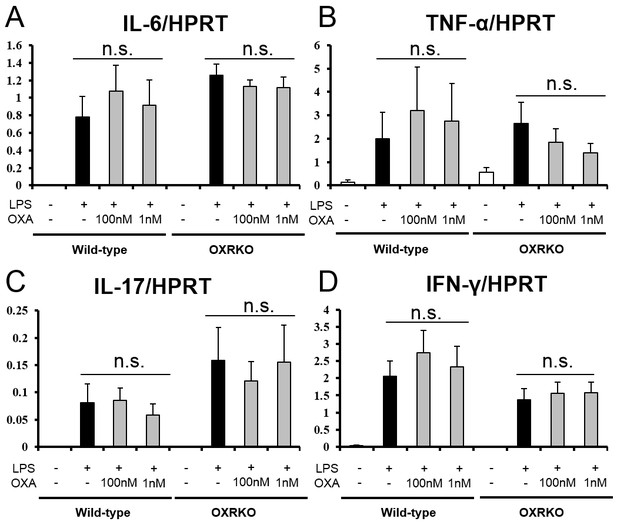
Effects of OXA on expressions of IL-6 (A), TNF-α (B), IL-17 (C), and IFN-γ (D) mRNA in cultured peritoneal macrophages from wild-type and OXRKO mice.
Hypoxanthine phosphoribosyl transferase-1 (HPRT) was used as an internal control (each group n = 6).
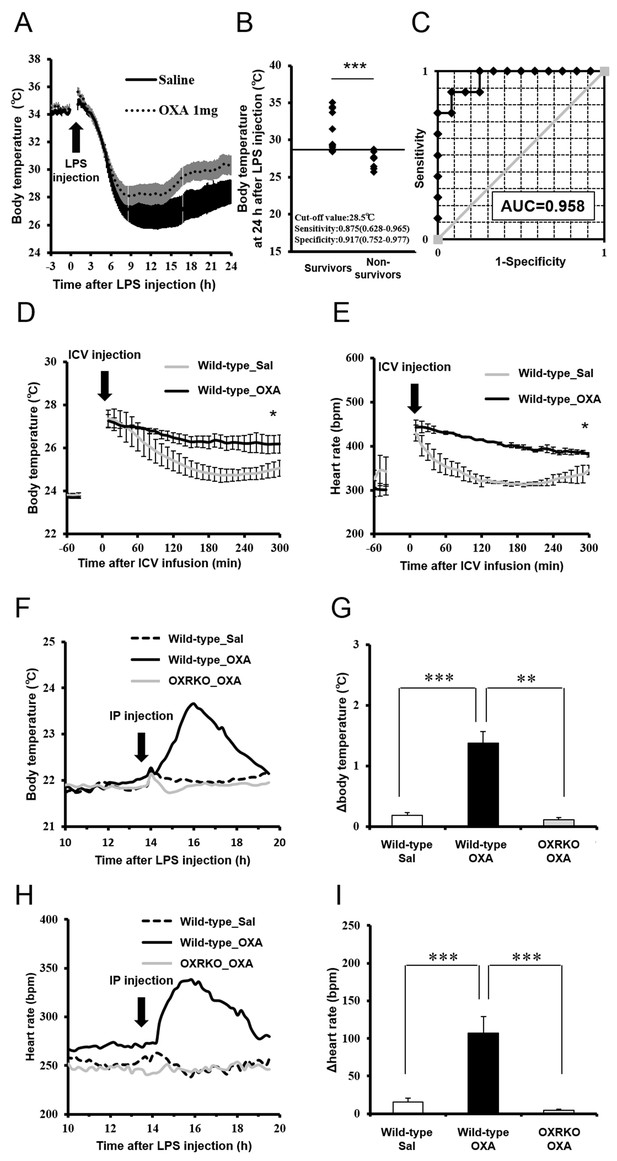
Effects of OXA on body temperature and heart rate in mice with endotoxin shock.
(A) The changes in body temperature of LPS-injected mice treated with OXA (1 mg/mouse/24 hr) or saline (each group n = 10). (B) The correlation between the survival and body temperature in mice with endotoxin shock (Survivor n = 12, Non-survivor n = 8, ***p<0.001). (C) Receiver operating characteristic (ROC) curve between survival and body temperature in mice with endotoxin shock. AUC: area under the curve. (D, E) Transient effects of bolus ICV-injected OXA (Wild-type_OXA) or saline (Wild-type_Sal) on body temperature (D) and heart rate (E) in LPS-injected wild-type mice (each group n = 4, *p<0.05). (F, H) Transient effects of bolus IP-injected OXA on body temperature (F) and heart rate (H) in mice with endotoxin shock. (G, I) IP injection of OXA (Wild-type_OXA) but not saline (Wild-type_Sal) increased body temperature (G) and heart rate (I) transiently in LPS-injected wild-type mice, but not in LPS-injected OXRKO mice (OXRKO_OXA) (each group n = 4, **p<0.01, ***p<0.001). Statistical significance assessed by unpaired t-test (B), 2-way ANOVA (D, E), and 1-way ANOVA coupled with the Bonferroni’s test (G, I). Data are replicated in at least three independent experiments.
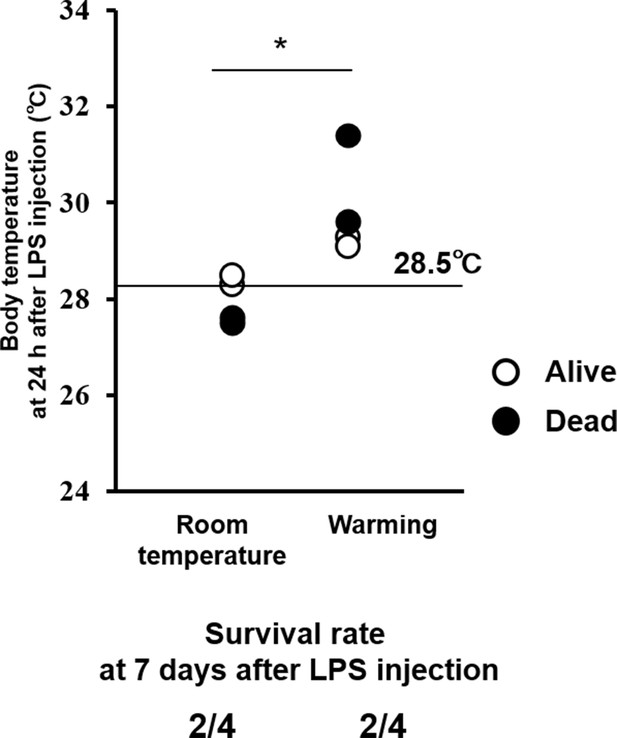
Effects of whole-body warming with a heat pad on body temperature (upper) and survival rate (lower) in mice with endotoxin shock.
Warming elevated body temperature at 24 hr after LPS injection, but did not improve the survival of mice with endotoxin shock, compared to room temperature exposure (each group n = 4, *p<0.05).
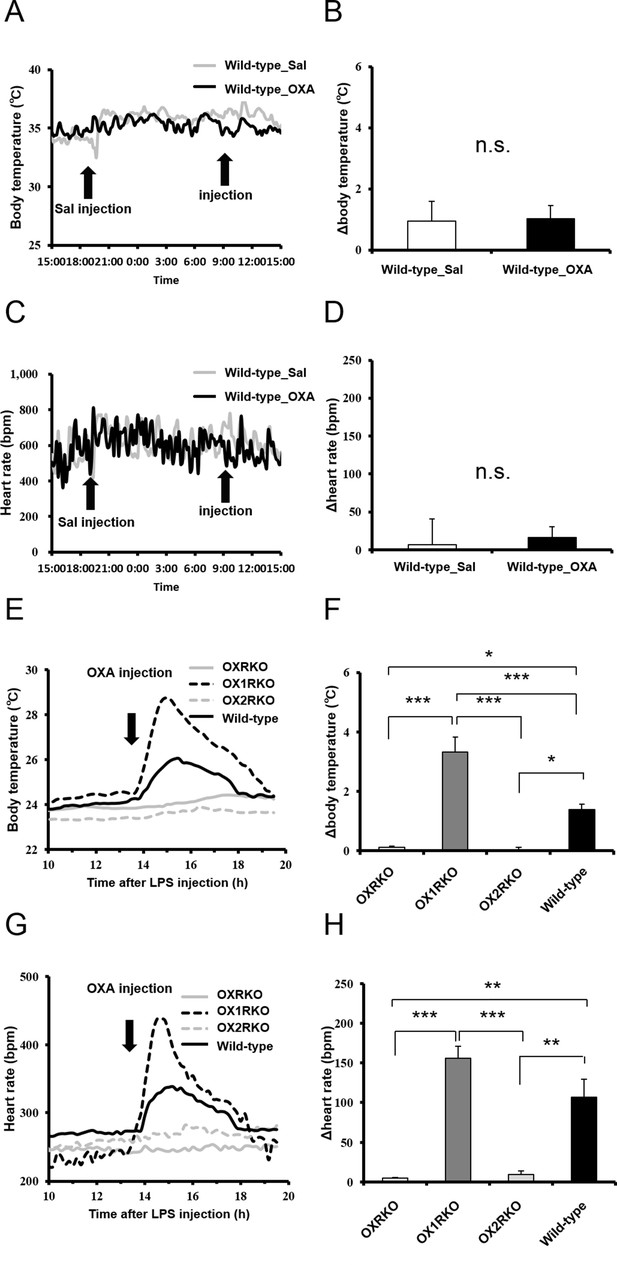
Transient effects of IP-injected OXA on body temperature and heart rate in healthy wild-type mice injected with saline (A, B, C, D) or in Hcrtr1-/-(OX1RKO) and Hcrtr2-/- (OX2RKO) mice with endotoxin shock (E, F, G, H).
IP injection of OXA had no effects on body temperature and heart rate in healthy wild-type mice. In OX2RKO mice, transient effects of OXA on body temperature and heart rate in endotoxin shock were canceled. In OX1RKO mice, the transient effect of OXA on body temperature in endotoxin shock was significantly potentiated. (each group n = 4, *p<0.05, **p<0.01, ***p<0.001).
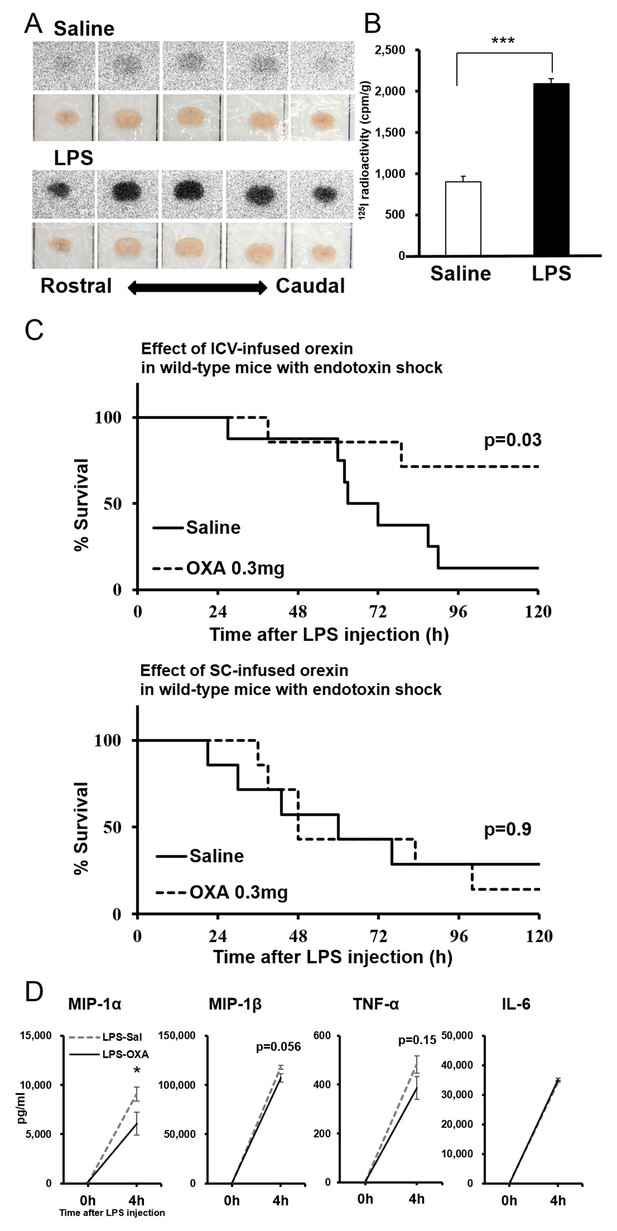
Direct action of OXA on the CNS in mice with endotoxin shock.
(A) [125I]OXA-autoradiography of 1 mm coronal brain sections from control (saline) and endotoxin shock (LPS) mice. The brains were removed without perfusion at 2 hr after intraperitoneal (IP) administration of [125I]OXA, and were fixed in 4% PFA overnight. The sections were exposed to imaging plates for five days, and then scanned by BAS-2500 (Fuji Film). (B) The levels of radioactivity in the whole brain from LPS- or saline-injected mice at 2 hr after IP administration of [125I]OXA (each group n = 4, ***p<0.001). (C) Kaplan-Meier survival curves of wild-type mice intracerebroventricularly (ICV, upper; saline: n = 8, OXA: n = 7) or subcutaneously (SC, lower; each group n = 7) administered with saline or OXA (0.3 mg/mouse/24 hr) before LPS injection. (D) Effects of ICV-administered OXA on the levels of MIP-1α, MIP-1β, TNF-α and IL-6 in serum at 4 hr after LPS injection (LPS-OXA), compared to saline treatment (LPS-Sal) (each group n = 8, *p<0.05). OXA administration (0.1 mg/mouse/4 hr) started 30 min before LPS injection. Data are presented as mean±s.e.m. Statistical significance assessed by unpaired t-test (B), Mantel Cox log-rank test (C), and 2-way ANOVA coupled with the Bonferroni’s test (D). Data are replicated in at least three independent experiments.
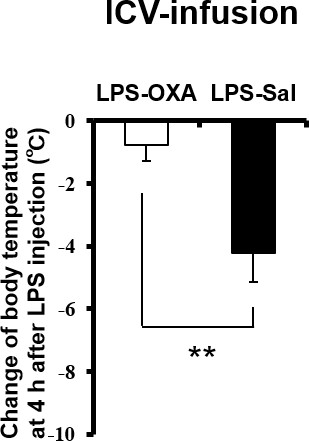
Effects of ICV-infused OXA on body temperature at 4 hr after LPS injection.
The changes of body temperature at 4 hr after LPS injection by ICV administration of OXA (0.3 mg/mouse/24 hr, LPS-OXA), compared to saline administration (LPS-Sal) (each group n = 8, **p<0.01).
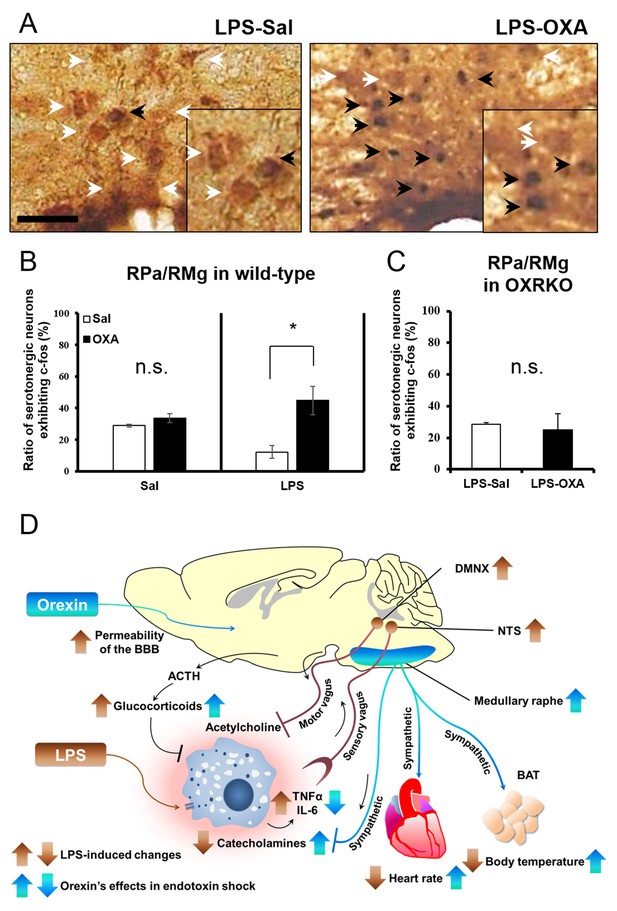
The hypothetical schema of multiple pathways by which OXA may improve survival in mice with LPS-induced endotoxin shock (see also main text).
(A) IP-administered OXA (LPS-OXA, right) but not saline (LPS-Sal, left) activated serotonergic neurons in the raphe pallidus nucleus and raphe magnus nucleus (RPa/RMg) in LPS-injected wild-type mice by immunohistochemistry using anti-c-fos and anti-5HT antibodies. Black arrows indicate double positive cells and white arrows indicate 5HT-single positive cells. (B, C) IP-administration of OXA significantly activated serotonergic neurons in RPa/RMg of LPS-injected wild-type mice, compared to saline administration (each group n = 4, *p<0.05) (B). There was no significant difference in the activities of serotonergic neurons in RPa/RMg between saline and OXA administration in healthy wild-type mice (B) or in OXRKO mice with endotoxin shock (C). Statistical significance assessed by unpaired t-test. Scale bar: 100 μm. Data are replicated in at least three independent experiments. (D) LPS activates macrophages via toll-like receptor 4 and produces TNF-α, IL-6, and other cytokines. These cytokines activate NTS and DMNX through sensory vagus nerves (Tracey, 2002). Activated motor vagus nerves regulate the inflammatory response and decrease heart rate and body temperature. Orexin sustains heart rate and body temperature through sympathetic nervous system by activating medullary raphe, and also regulates the inflammatory response by central actions. Orexin thus improves the survival through a multitude of pathways, including neuroendocrine and autonomic nervous systems. NTS: nucleus tractus solitaries. DMNX: dorsal motor nucleus of the vagus.
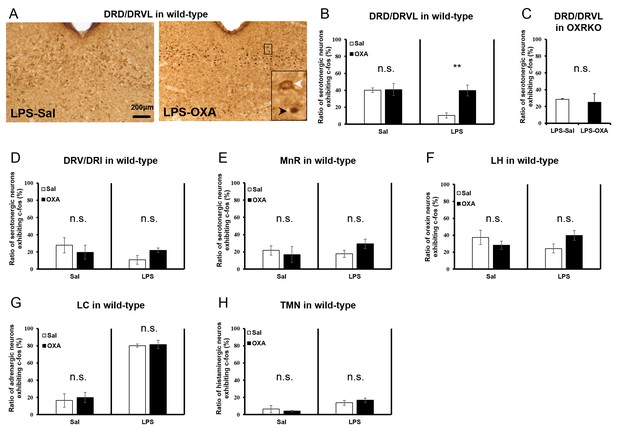
Systematic survey of orexin’s targets in the CNS of mice with endotoxin shock.
(A) Typical photographs of double staining for c-fos and 5HT in DRD/DRVL from LPS-injected mice treated with saline (LPS-Sal, left) or OXA (LPS-OXA, right). (B, C) The percentage of c-fos positive serotonergic neurons in DRD/DRVL from LPS-injected mice treated with saline or OXA, form saline-injected normal mice treated with saline or OXA (B) and from LPS-injected OXRKO mice treated with saline or OXA (C). (D, E) The percentage of c-fos positive serotonergic neurons in DRV/DRI (D) and in MnR (E) from LPS-injected mice treated with saline or OXA and from normal mice treated with saline or OXA. (F) The percentage of c-fos positive orexin neurons in LH from LPS-injected mice treated with saline or OXA and from normal mice treated with saline or OXA. (G, H) The percentage of c-fos positive noradrenergic neurons in LC (G) and histaminergic neurons in TMN (H) from LPS-injected mice treated with saline or OXA and from normal mice treated with saline or OXA. (B–H each group n = 4, **p<0.01). DRD: dorsal raphe nucleus, dorsal part; DRVL: dorsal raphe nucleus, ventrolateral part, DRV: dorsal raphe nucleus, ventral part, DRI: dorsal raphe nucleus, interfascicular part, LH: lateral hypothalamus, LC: locus coeruleus, TMN: tuberomammillary nucleus.
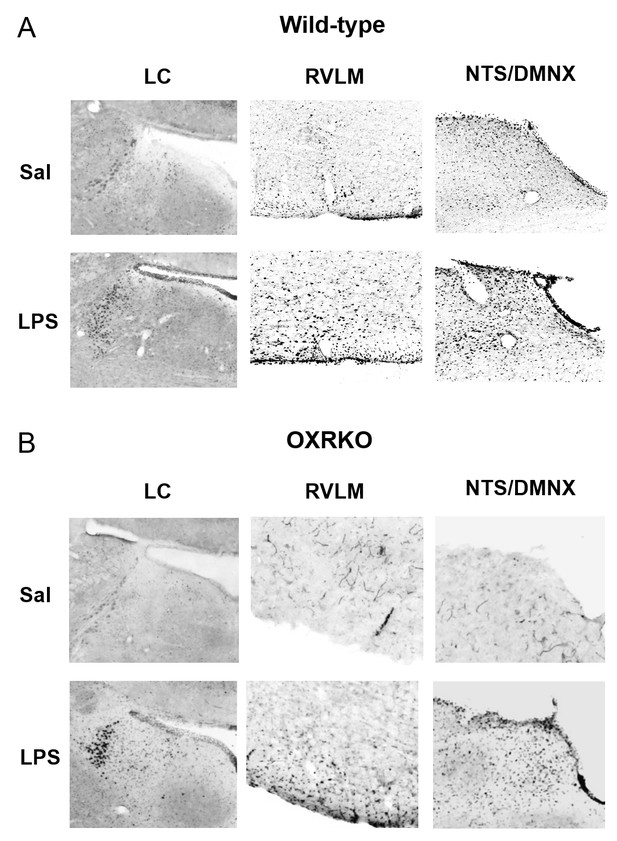
Activation of neurons in the LC, RVLM, NTS and DMNX at 3 hr after LPS injection in wild-type (A) or OXRKO (B) mice.
Typical photographs of Fos staining in LC (left), RVLM (middle), NTS and DMNX (right) from wild-type mice or OXRKO mice injected with saline or LPS. LC: locus coeruleus. RVLM: rostral ventrolateral medulla. NTS: nucleus tractus solitaries. DMNX: dorsal motor nucleus of the vagus.





