Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation
Figures
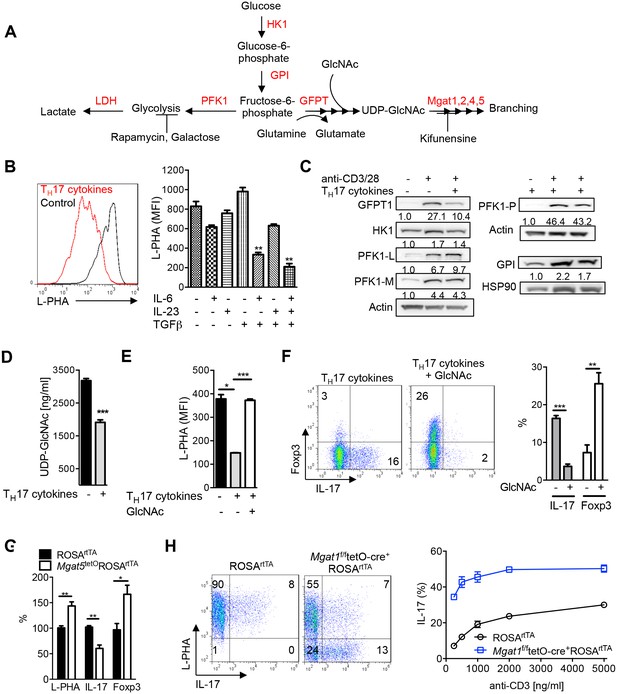
N-glycan branching controls TH17 versus iTreg cell fate.
(A) Fructose 6-phosphate and glutamine may be metabolized by glycolysis and glutaminolysis, respectively, or enter the hexosamine pathway to supply UDP-GlcNAc to the Golgi branching enzymes Mgat1, 2, 4 and 5. HK: hexokinase, GPI: glucose-6-phosphate isomerase, PFK1: phosphofructokinase1, LDH: Lactate dehydrogenase, GFPT: glutamine-fructose-6-phosphate transaminase. (B–H) Flow cytometry (B,E–H), Western blot (C) and LC-MS/MS (D) analysis of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 for 4 days (B,E–H) or 3 days (C,D) with TH17 inducing conditions (TGFβ+IL-6+IL-23) or as indicated. PFK1-L (liver), PFK1-P (platelet), PFK1-M (muscle). (G) Co-incubation with doxycycline in vitro. (H) Doxycycline treatment in vivo, with Mgat1f/ftetO-Cre+ROSArtTA cells in right panel gated on L-PHA− population. (B,E–H) gated on CD4+. (B,D–H) Unpaired two tailed t-test with Welch’s (E) and Bonferroni corrections (B,E). **p<0.01; ***p<0.001. Data are mean ± s.e.m of triplicate cultures and representative of n ≥ 3 experiments. MFI, mean fluorescence intensity.
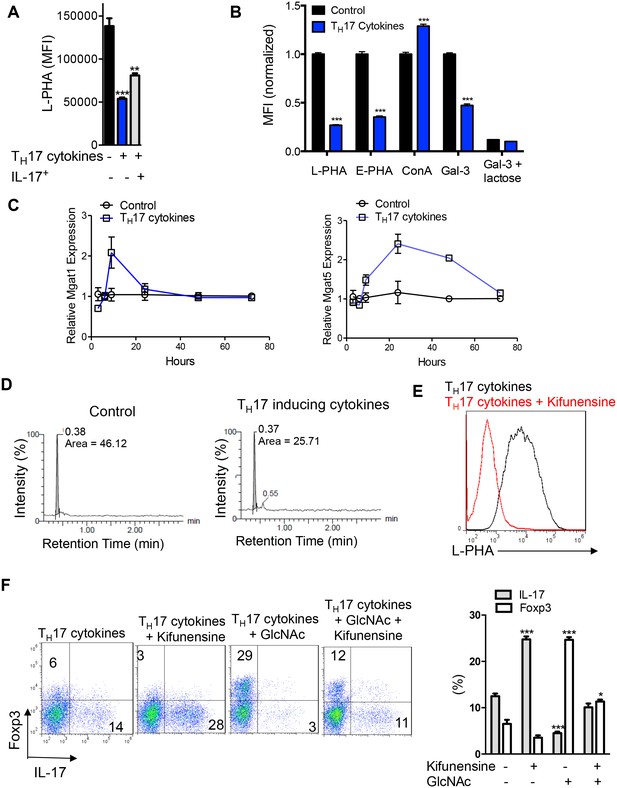
N-glycan branching controls TH17 versus iTreg cell fate.
(A–F) Flow cytometry (A,B,E,F), real-time qPCR (C) and UDP-GlcNAc LC-MS/MS analysis (D) of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17 inducing conditions (TGFβ+IL-6+IL-23) for 3 days (D) or 4 days (A,B,E,F) or as indicated (C). (A) gated on CD4+IL-17A- or CD4+IL-17A+ as indicated. (B,E,F), gated on CD4. (B) Lactose (50 mM) is an inhibitor of galectin binding to branched N-glycans. (A,B,F) Unpaired two tailed t-test (B) with Bonferroni corrections (A,F). *p<0.05; **p<0.01; ***p<0.001. Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
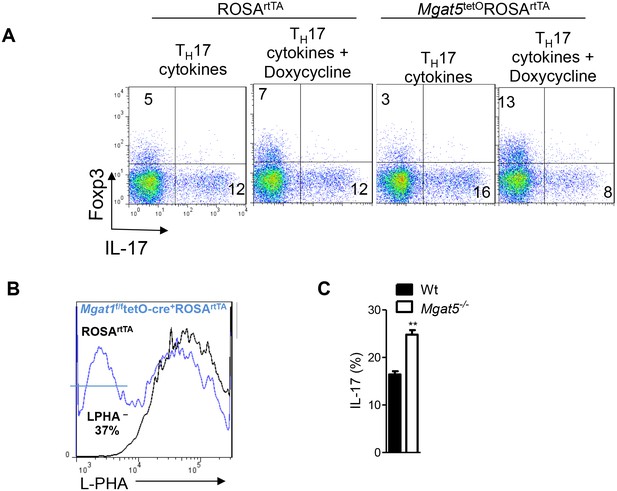
N-glycan branching controls TH17 versus iTreg cell fate.
(A–C) Flow cytometry of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17 inducing conditions (TGFβ+IL-6+IL-23) or as indicated for 4 days. Gated on CD4+. (C) Unpaired two tailed with Bonferroni corrections. *p<0.05; **p<0.01; ***p<0.001. Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
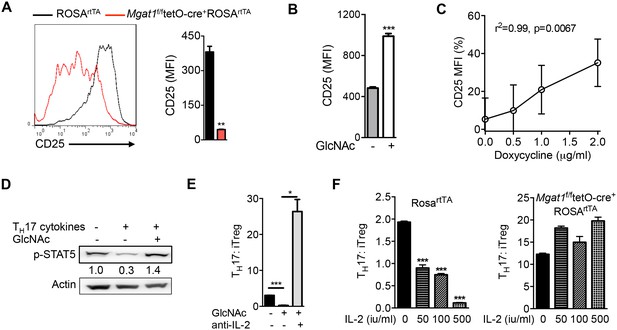
N-glycan branching controls TH17 versus iTreg cell fate via IL-2Rα (CD25).
(A–F) Flow cytometry (A–C,E,F) and Western blot (D) analysis of mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17-inducing conditions (TGFβ+IL-6+IL-23) for 3 days (D) or 4 days (A–C,E,F); cells gated on CD4+. (A,F) Doxycycline treatment in vivo with Mgat1f/ftetO-Cre+ROSArtTA cells gated on L-PHA− population. (C) CD25 MFI in Mgat5tetOROSArtTA CD4+ T cells normalized to ROSrtTA CD4+ T cells, both treated in vitro with doxycycline. (E,F) ratio of IL-17A+ to FoxP3+ CD4+ cells. (A,B,E,F) Unpaired two-tailed t-test with Welch’s (A,E) and Bonferroni corrections (E,F). (C) Linear regression. *p<0.05; **p<0.01; ***p<0.001. Data are mean ± s.e.m of triplicate cultures and representative of n ≥ 3 experiments. MFI, mean fluorescence intensity.
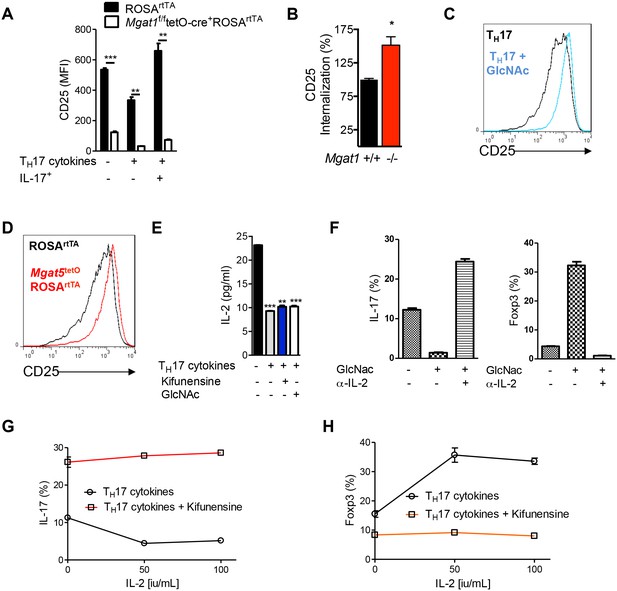
N-glycan branching controls TH17 versus iTreg cell fate via IL-2Rα (CD25).
(A–D,F–H) Flow cytometry of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17 inducing conditions (TGFβ+IL-6+IL-23) or as indicated for 4 days. (D) Co-incubation with doxycycline in vitro. (A–C,D,F–H) gated on CD4+. (A,B) Mice treated with doxycycline in vivo, Mgat1f/ftetO-Cre+ROSArtTA gated on L-PHA- cells. (A) Gated on CD4+IL-17A- or CD4+IL-17A+ as indicated. (B) Endocytosis analyzed by flow cytometry for CD25 internalization. Internalized percentage (MFI (internalized) / MFI (total) × 100%), normalized to control. (D) ELISA analysis of supernatant from purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17-inducing conditions (TGFβ+IL-6+IL-23) as indicated for 4 days. (A,B,E) Unpaired two-tailed t-test with Welch’s (A) and Bonferroni corrections (E). *p<0.05; **p<0.01; ***p<0.001. Data are mean ± s.e.m and n = 3, except n = 2 for (B). MFI, mean fluorescence intensity.
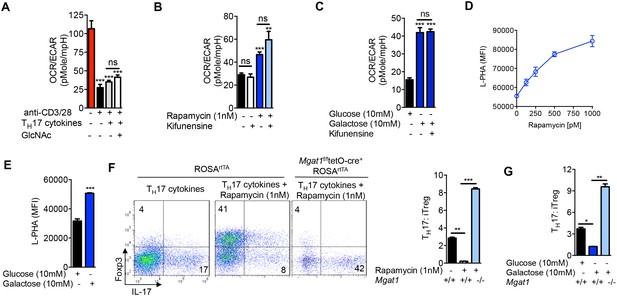
Glycolysis promotes TH17 over iTreg cell fate by inhibiting N-glycan branching.
(A–C) Ratio of the oxygen consumption rate (OCR) to the extracellular acidification rate (ECAR) of purified splenic CD4+ T cells at rest or activated with anti-CD3+anti-CD28 for 2 days with TH17 cytokines (TGFβ+IL-6+IL-23) or as indicated. (D–G) Flow cytometry analysis of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 for 4 days under TH17-inducing conditions (TGFβ+IL-6+IL-23). (F (right panel), G) ratio of IL-17A+ to Foxp3+ CD4+ T cells, with Mgat1f/ftetO-Cre+ROSArtTA cells gated on L-PHA- population. *p<0.05; **p<0.01; ***p<0.001. NS, not significant. (A–G), Unpaired two-tailed t-test with Welch's (B,C,F,G) and Bonferroni correction (A–C,F,G). Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
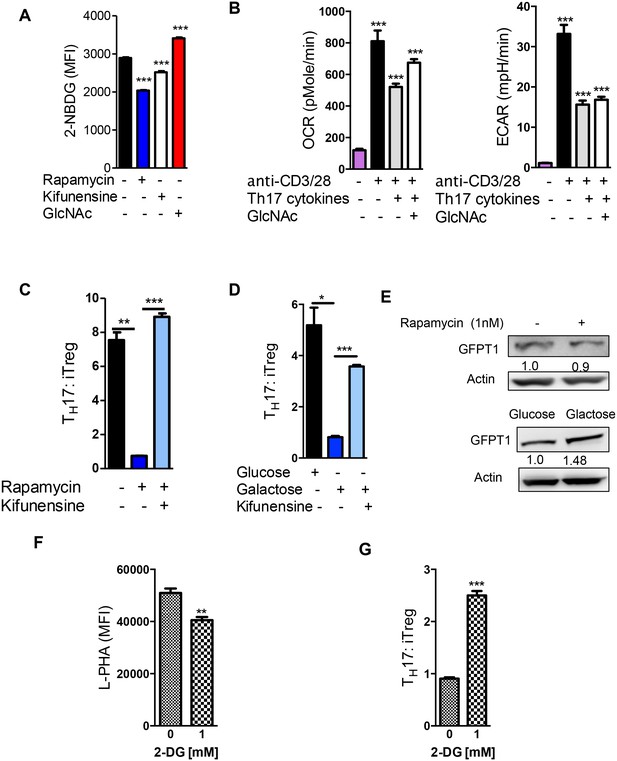
Glycolysis promotes TH17 over iTreg cell fate by inhibiting N-glycan branching.
(A) Flow cytometry analysis of purified splenic CD4+ T cells activated with anti-CD3+ anti-CD28 for 2 days with TH17 cytokines and as indicated for 1 hr with 2-NBDG. (B) Oxygen Consumption Rate (OCR) and the Extracellular Acidification Rate (ECAR) of purified splenic CD4+ T cells at rest or activated with anti-CD3+anti-CD28 for 2 days with/without TH17 cytokines (TGFβ+IL-6+IL-23) as indicated. (C–G) Flow cytometry (C,D,F,G) and Western blot (E) analysis of purified mouse splenic CD4+ T-cells activated with anti-CD3+anti-CD28 under TH17-inducing conditions (TGFβ+IL-6+IL-23). Gated on CD4+ T cells. *p<0.05; **p<0.01; ***p<0.001. (A–D,F,G) Unpaired two-tailed t-test with Welch's (B–D) and Bonferroni correction (A–D). Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
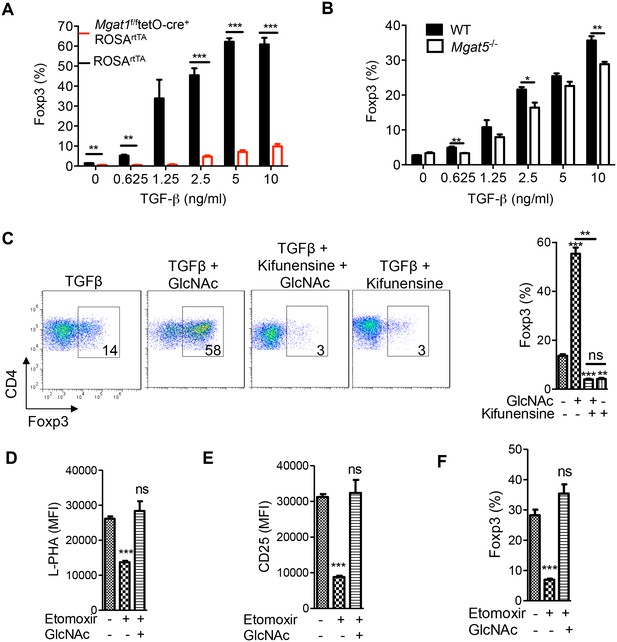
Oxidative phosphorylation promotes iTreg generation by enhancing N-glycan branching.
(A–F) Flow cytometry of purified splenic CD4+ T cells activated with anti-CD3+anti-CD28 and TGFβ1 for 4 days; gated on CD4+. (A), Mgat1f/ftetO-Cre+ROSArtTA cells gated on L-PHA- population from mice treated with doxycycline in vivo. *p<0.05; **p<0.01; ***p<0.001. (A–F) Unpaired two-tailed t-test with Bonferroni (C–F) and Welch’s (C,E,F) correction. Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
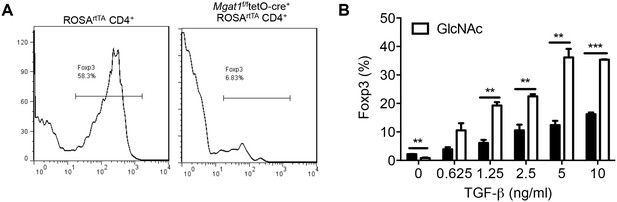
Oxidative phosphorylation promotes iTreg generation by enhancing N-glycan branching.
(A,B) Flow cytometry of purified splenic CD4+ T cells activated with anti-CD3+anti-CD28 and TGFβ1 for 4 days; gated on CD4+. (A) Mgat1f/ftetO-Cre+ROSArtTA cells gated on L-PHA- population from mice treated with doxycycline in vivo. *p<0.05; **p<0.01; ***p<0.001. (B) Unpaired two-tailed t-test; data are mean ± s.e.m. n = 3.
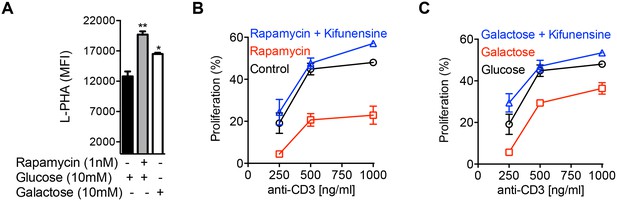
Glycolysis promotes T cell growth by inhibiting N-glycan branching.
(A–C) Flow cytometry of purified splenic CD4+ T cells activated with anti-CD3 for 3 days, gated on CD4+. (B,C) Proliferation measured by CFSE dilution. *p<0.05; **p<0.01; ***p<0.001. (A) Unpaired two-tailed t-test with Bonferroni correction. Data are mean ± s.e.m of triplicate cultures and representative of n = 3 experiments. MFI, mean fluorescence intensity.
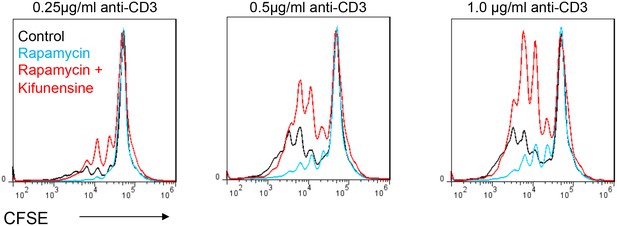
Glycolysis promotes T cell growth by inhibiting N-glycan branching.
Flow cytometry of CFSE-labeled splenic CD4+ T cells activated with anti-CD3 for 3 days, gated on CD4+. n = 3.
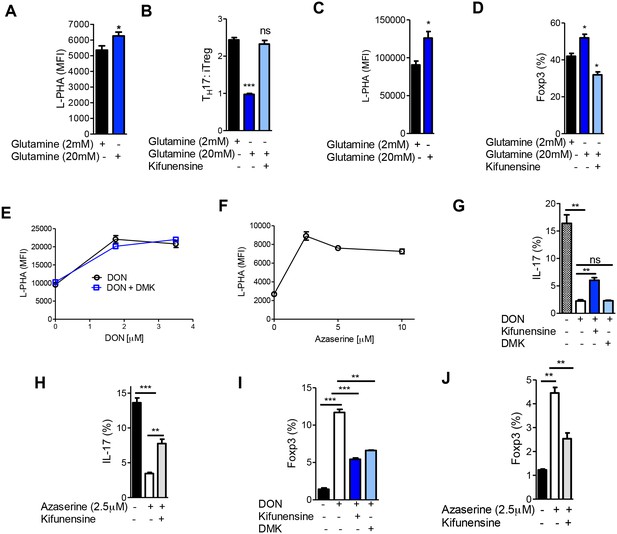
Glutaminolysis promotes TH17 over iTreg cell fate by inhibiting N-glycan branching.
(A–J) Flow cytometry of purified splenic CD4+ T cells activated with anti-CD3+anti-CD28 under TH17-inducing conditions (TGFβ+IL-6+IL-23) (A,B,E–J) or in the presence of TGFβ (C,D). Gated on CD4+. *p<0.05; **p<0.01; ***p<0.001. (A–D,G–J) Unpaired one-tailed t-test with Welch's (G,I,J) and Bonferroni (B,D,G–J) correction. Data are mean ± s.e.m and n = 3. MFI, mean fluorescence intensity.
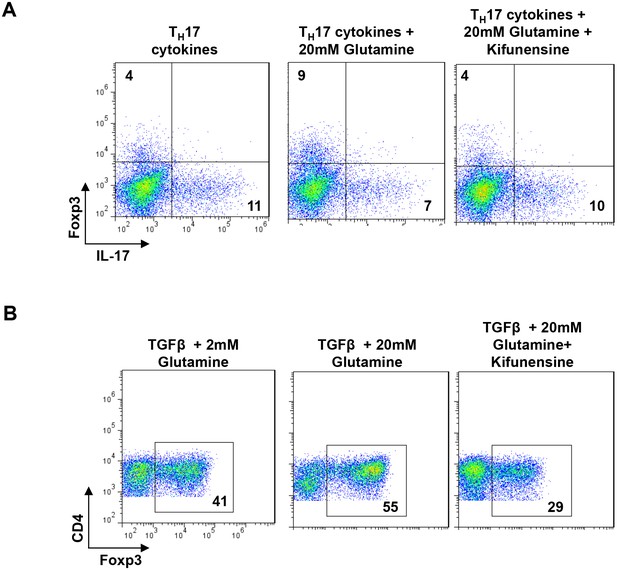
Glutaminolysis promotes TH17 over iTreg cell fate by inhibiting N-glycan branching.
(A,B) Flow cytometry of purified splenic CD4+ T cells activated with anti-CD3+anti-CD28 under TH17-inducing conditions (TGFβ+IL-6+IL-23) (A) or in the presence of TGFβ (B). Gated on CD4+. n = 3.





